- Joined
- Nov 26, 2013
- Messages
- 7,611
- Reaction score
- 14,409
Opened 5.1 in Excel 2007, no FALSEs
Added a line (and misspelled Munich)
Deleted the leftmost col. Still no FALSE.
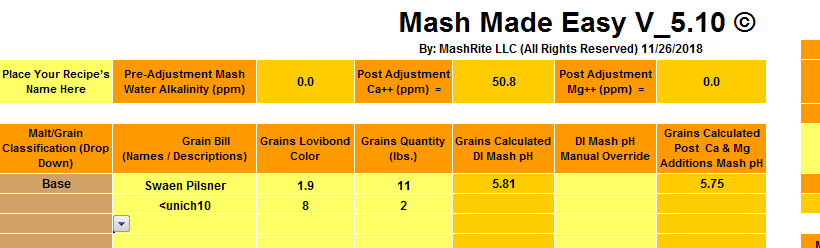
Edit: same thing in Excel 2016. No FALSEs. (Falsies?)
Added a line (and misspelled Munich)
Deleted the leftmost col. Still no FALSE.

Edit: same thing in Excel 2016. No FALSEs. (Falsies?)