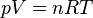The last batch I bottled was a Haus pale ale, and when I got down to the last bit in the bottling bucket it wasn't enough for a full 12oz bottle. So I filled a little over half of a bottle. Not knowing better I figured what the hell I have the caps I'll just throw one on it and let it sit and see how it carbs up.
Take it from me, don't cap a bottle with that much head space.
Oh my....it was SOOOOO heavily carbonated.
I'm assuming due to all the compressible air space, the CO2 production went wild.
Upon opening the bottle there was a loud / long pressure release Psssssssst.
The bottle didn't gush so I was hopefull. I got out a glass and SLOWLY poured just a small bit into the glass, barely an ounce. As soon as the beer touched the glass it filled the entire glass with foam. Kind of like that spray foam you use to seal houses...
Need less to say I let it sit out over night and drank it the next day and it still had carbonation in it! The flavor was still quite over powered by the carbonation.
Take it from me, don't cap a bottle with that much head space.
Oh my....it was SOOOOO heavily carbonated.
I'm assuming due to all the compressible air space, the CO2 production went wild.
Upon opening the bottle there was a loud / long pressure release Psssssssst.
The bottle didn't gush so I was hopefull. I got out a glass and SLOWLY poured just a small bit into the glass, barely an ounce. As soon as the beer touched the glass it filled the entire glass with foam. Kind of like that spray foam you use to seal houses...
Need less to say I let it sit out over night and drank it the next day and it still had carbonation in it! The flavor was still quite over powered by the carbonation.