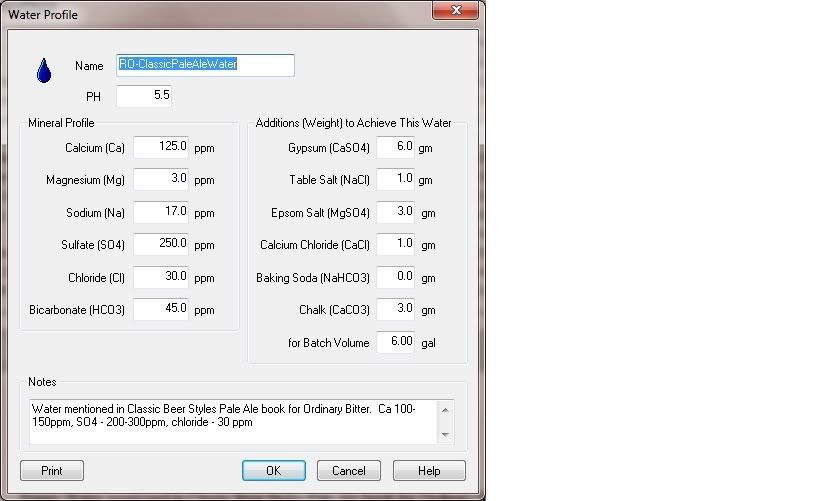
I stumbled upon this PDf that list a ton of cities and how to make their water profile from Deionized water.
http://ajdel.wetnewf.org:81/Brewing_articles/Recipes.pdf
"The pages which follow contain recipes for formulation of water with the properties of the indicated cities from calcium carbonate, calcium chloride, sodium chloride, sodium bicarbonate, calcium sulfate, and magnesium sulfate. Where the use of magnesium carbonate appreciably improves the accuracy of a formulation we give separate recipes: one with magnesium carbonate and one without. None of these formulations require the addition of acid but it is assumed that calcium carbonate (chalk) and magnesium carbonate (where used) is dissolved by sparging the
entire volume of water being prepared with carbon dioxide or by first dissoving the chalk in carbonic acid made by pressurizing cold water in a cornelius keg with CO2 and shaking. All the recipes use deionized water as the base water.
The formulations are designed to get the concentrations of all dissolved species as close to the desired levels as possible. As we indicated in Part III it is often not possible to get very close at all. We recommend choice of the profile for a particular city which gives the best, in the sense of closest approximation, synthesis. Salt quantities specified are in milligrams per liter.
The individual recipies are output from the FORTRAN program of Appendix J. Individual items should be familiar. At the top of the page we list the city name and im balance information in terms of the pH required to acheive electrical neutrality where the profile can be balanced at reasonable pH and, where it cannot, the imbalance at pH 8.4. Note that if pH has been set to 8.4 the profile synthesized will be electrically balanced as all real water samples must be but that one or more ions will be in appreciable error."
Here is an example:
Target City: London2 Base Water: Deionized
Balancing pH 10.6957 is greater than 8.40 and is thus set to 8.40
Net charge (imbalance) at this pH: 2.0915 mEq/L
SALTS ADDED FOR THIS SYNTHESIS:
Sodium Chloride : 1.65 mg/L
Calcium Sulfate Dihydrate : 44.72 mg/L
Calcium Chloride Dihydrate : 42.04 mg/L
Magnesium Sulfate Heptahydrate : 48.67 mg/L
Calcium Carbonate : 107.10 mg/L
Magnesium Carbonate : 0.00 mg/L
Sodium Bicarbonate : 49.15 mg/L
Carbonic Acid : 2.10 mEq/L
COMPARISON OF TARGET AND SYNTHESIS:
TARGET SYNTHESIS pRatio Pct Err
London2
pH : 8.40 8.40
f1 : 0.0080 0.0080
f2 : 0.9769 0.9771
f3 : 0.0152 0.0149
Ionic Strength : 7.3948 6.5339
pfm : 0.0403 0.0381
Carbonates* : 2.0492 2.7029 mM/L +0.1203 +31.90%
Calcium* : 90.00 64.76 mg/L -0.1429 -28.05%
Carbonic : 1.01 1.34 mg/L +0.1226 +32.61%
Bicarbonate : 122.17 161.18 mg/L +0.1203 +31.93%
Carbonate : 1.86 2.42 mg/K +0.1136 +29.91%
Alkalinity (as CaCO3): 105.08 137.64 mg/L
Chloride* : 20.00 21.27 mg/L +0.0268 +6.37%
Magnesium* : 5.00 4.80 mg/L -0.0178 -4.02%
Sodium* : 15.00 14.10 mg/L -0.0269 -6.00%
Sulfate* : 40.00 43.92 mg/L +0.0406 +9.80%
Nitrate : 0.00 0.00 mg/L
RMS Log Error (Items with *): 0.07990 Corresponding % 20.1979
pHs : 7.40 7.41
Saturated WRT CaCO3? : Yes Yes
Langelier Index : 1.00 0.99 SI < 0 ~ Corrosion; SI > 0 ~ Occlusion
Ryznar Index : 6.40 6.42 RI < 6 ~ Occlusion; RI > 7 ~ Corrosion
pHe : 8.60 8.73
Saturated WRT CO2? : Yes Yes
CO2 Equilibrium Index: 0.20 0.33 EI < 0 ~ Gains CO2; EI > 0 ~ Loses CO2
Residual Alkalinity : 37.98 88.66 mg/L as CaCO3