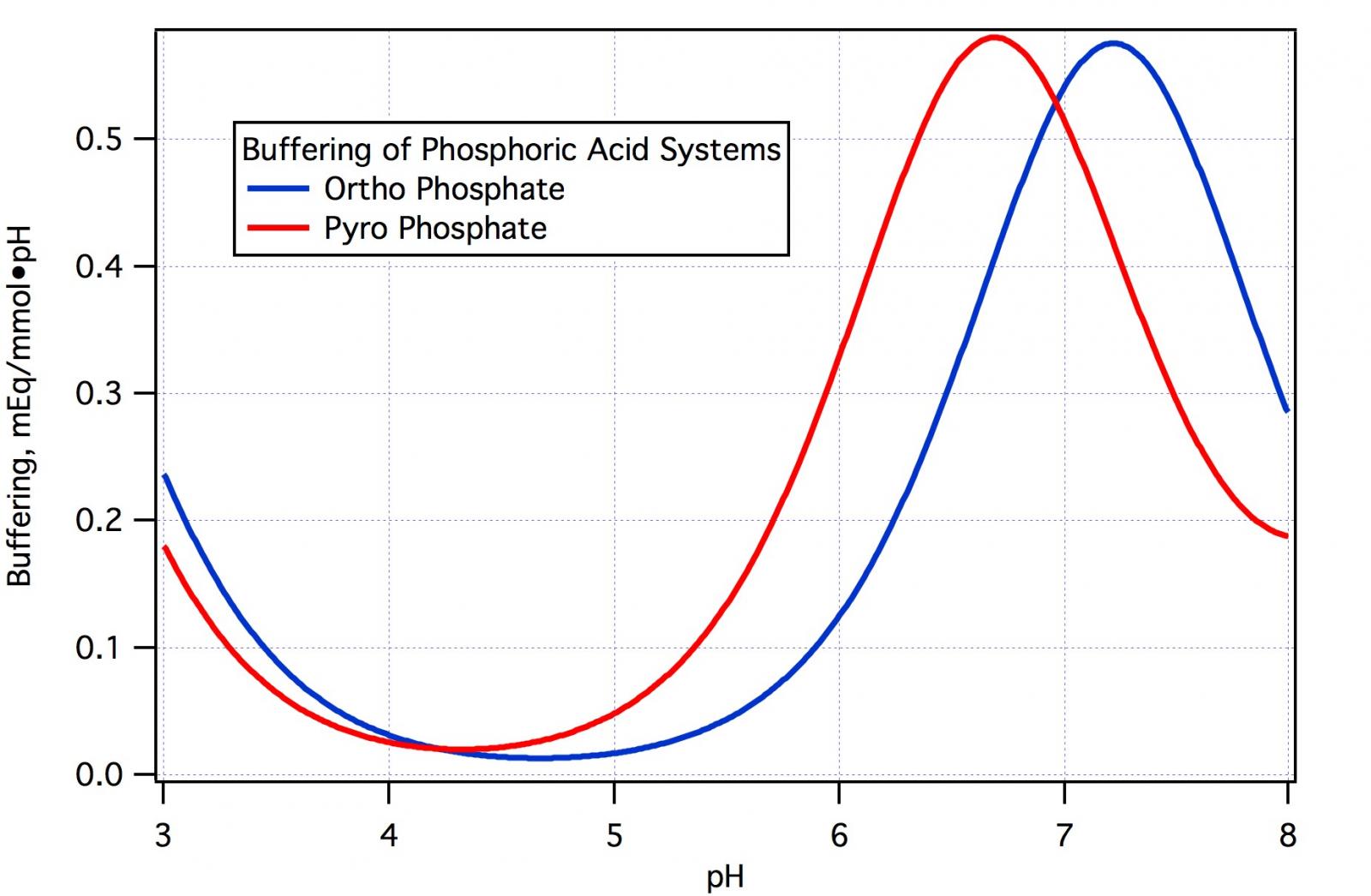
Not a chemist but the pH5.2 threads have me curious if it's even possible to construct a dry food grade buffer to a specific pH within the recommended mash range 5.2-5.6? Are the necessary acids even able to be dried? Are they stable in such a state?
(I know the theory of a one size fits all buffer is flawed but just humor me anyway.)
(I know the theory of a one size fits all buffer is flawed but just humor me anyway.)