If you're no sparging, all the water you use is mash water, none is sparge. All the salts would be stirred into the mash.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Water Modification Videos, TH's Spreadsheet
- Thread starter Bobby_M
- Start date

Help Support Homebrew Talk - Beer, Wine, Mead, & Cider Brewing Discussion Forum:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
kal
Well-Known Member
Kal, if you list your whole profile, I can play around to see if I can get something you can work with.
Thanks Bobby!
I'll give my water numbers using my last beer as an example: A Blonde Ale with SRM of 5. I have no issues with getting to Moser Pale Ale style numbers. It's the lighter beers like this (Helles, american lagers) that I have issues with.
My water's soft.
My intentions in water modifications for this lighter coloured/style beer was:
(1) Keep the additions fairly light but still try and get all of the numbers into the recommended range (within reason)
(2) Create a balanced Cl:S04 ratio
Here's what I did:
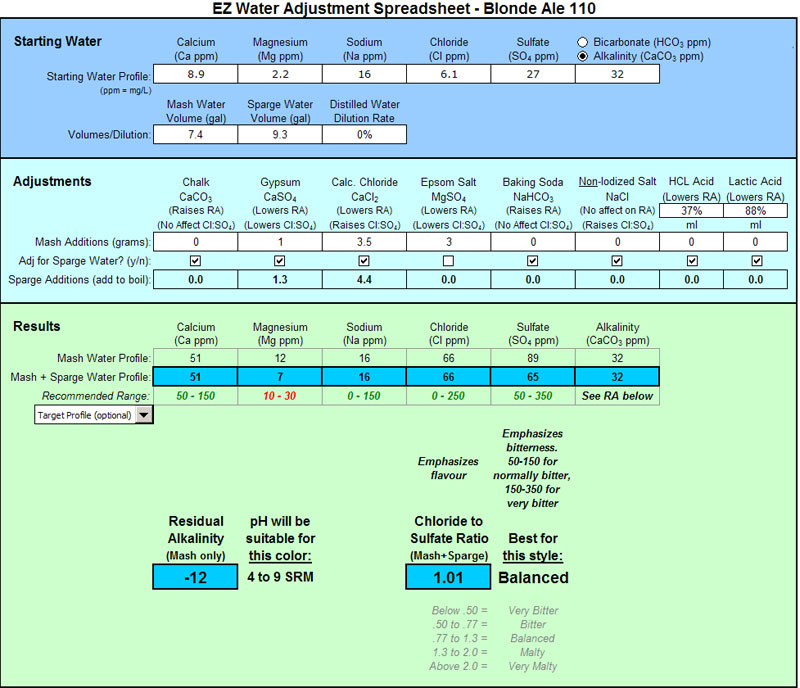
Mg is low as you can see. I keep hearing that many people don't want to add extra Epsom Salt in the kettle so I left it out. I suppose I could have included the Epsom Salt in the sparge water as well and then upped the CaCl to raise Cl as follows:
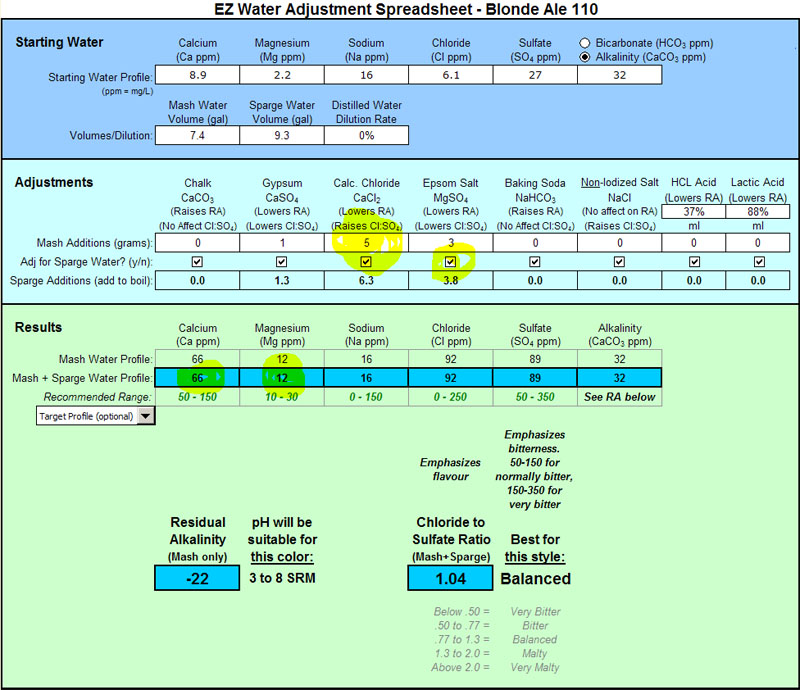
This way I would have hit the recommended range and had a balanced Cl:S04.
I guess my question really is: What sort of targets would you guys want to aim for for something like a Blonde Ale done right to style guidlines (American 2-row, a very little bit of Crystal 15L, fermented with WLP001/WY1056 at 67F)?
I'm doing a Helles next week to style and figure I should go even more towards the "malty" side of Cl:S04 ratio.
Thanks!
Kal
I think both are OK. No harm in adding epsom to the boil that I can tell.
According to the profile in the EZ calc, Munich is still on the bitter side of Cl:SO4, but not overly so. I think balanced to 1.25 would be good.
According to the profile in the EZ calc, Munich is still on the bitter side of Cl:SO4, but not overly so. I think balanced to 1.25 would be good.
If you're no sparging, all the water you use is mash water, none is sparge. All the salts would be stirred into the mash.
Thanks again. I should have seen that in Daddymem's post about BIAB. Just ordered my minerals and plan to adjust close to Munich profile for an upcoming Dunkelweizen. Before that, I'm gonna try a Marris Otter SMaSH w/o adjustments or minor adjustments just to increase my Ca and Mg a tad. I've had good results with Ed Wort's Pale Ale without adjustments. Think the SMaSH will be ok?
kal
Well-Known Member
I think both are OK. No harm in adding epsom to the boil that I can tell.
According to the profile in the EZ calc, Munich is still on the bitter side of Cl:SO4, but not overly so. I think balanced to 1.25 would be good.
Thanks Bobby. In reading about TH's spreadsheet it's been mentioned a few times that people prefer to keep salts out of the boil (esp. MgS04), which is why the checkboxes are added. Not 100% sure why.
Kal
The two salts I regularly leave out of the boil are Chalk and Baking Soda. They are typically only good for raising RA for the mash pH. Also, if you need to use Gypsum and others to drastically lower RA, you'll likely get all the SO4 you'd need out of the mash addition so you wouldn't want to layer more on top for the boil.
garretto
Well-Known Member
But is there any thing wrong with adding them to the boil? I'm doing my first salt addition on my next batch and I'm trying to keep sane by trying to keep things simple the first time.
I guess my question restated would be: Are there any negative effects of boiling chalk and/or baking soda? (Given that I follow what EZ water calculator determines how much I add)
I guess my question restated would be: Are there any negative effects of boiling chalk and/or baking soda? (Given that I follow what EZ water calculator determines how much I add)
No, the only potential issue is going overboard on the Ca, Na, and HCO3. I think the best bet would be to fill out the EZ sheet, and copy and paste the data from the "raw data" worksheet. We can all weigh in on your plan for a sanity check.
garretto
Well-Known Member
I guess I'm not so much asking for help with the salt additions (although I definitely would love some help, which is while I'll go ahead and post them below) as much as I'm trying to ask a more general question of the effects of adding salts to the boil.
Originally, I was just going to follow the EZ sheet gram for gram after I got my amounts correct, but then I watched the video about unchecking the box for boil as well as read that some leave certain salts out of the boil.
So is it generally just Ca, Na, and HCO3 you need to worry about? I imagine too much of any individual element (Na, Ca,...) is bad so is there a general rule of thumb like don't go above 7 grams of any salt? I realize the feedback from my info below may possibly answer a few questions.
This is for a Pale Ale
Starting Water (ppm):
Ca: 4
Mg: 1
Na: 6
Cl: 2.5
SO4: 1
CaCO3: 17
Mash / Sparge Vol (gal): 4 / 4.5
Dilution Rate: 0%
Adjustments (grams) Mash / Boil Kettle:
CaCO3: 0 / 0
CaSO4: 7 / 7.875
CaCl2: 0 / 0
MgSO4: 3.5 / 3.9375
NaHCO3: 0 / 0
NaCl: 1 / 1.125
HCL Acid: 0 / 0
Lactic Acid: 0 / 0
Mash Water / Total water (ppm):
Ca: 109 / 109
Mg: 23 / 23
Na: 32 / 32
Cl: 43 / 43
SO4: 349 / 349
CaCO3: 17 / 17
RA (mash only): -74 (0 to 4 SRM)
Cl to SO4 (total water): 0.12 (Very Bitter)
Thanks for taking a look at this.
Originally, I was just going to follow the EZ sheet gram for gram after I got my amounts correct, but then I watched the video about unchecking the box for boil as well as read that some leave certain salts out of the boil.
So is it generally just Ca, Na, and HCO3 you need to worry about? I imagine too much of any individual element (Na, Ca,...) is bad so is there a general rule of thumb like don't go above 7 grams of any salt? I realize the feedback from my info below may possibly answer a few questions.
This is for a Pale Ale
Starting Water (ppm):
Ca: 4
Mg: 1
Na: 6
Cl: 2.5
SO4: 1
CaCO3: 17
Mash / Sparge Vol (gal): 4 / 4.5
Dilution Rate: 0%
Adjustments (grams) Mash / Boil Kettle:
CaCO3: 0 / 0
CaSO4: 7 / 7.875
CaCl2: 0 / 0
MgSO4: 3.5 / 3.9375
NaHCO3: 0 / 0
NaCl: 1 / 1.125
HCL Acid: 0 / 0
Lactic Acid: 0 / 0
Mash Water / Total water (ppm):
Ca: 109 / 109
Mg: 23 / 23
Na: 32 / 32
Cl: 43 / 43
SO4: 349 / 349
CaCO3: 17 / 17
RA (mash only): -74 (0 to 4 SRM)
Cl to SO4 (total water): 0.12 (Very Bitter)
Thanks for taking a look at this.
garretto
Well-Known Member
Hey Kal, I just noticed your little notes below your sulfate about 50-150 being normally bitter and up to 350 being really bitter. How's that range working for you?
I forget if I have read about that in Palmer or not.
I forget if I have read about that in Palmer or not.
You have a situation where there's no harm in adding any of those salts to the boil since they are flavor affecting. Let's say you were adding a bunch of gypsum to the mash for Ca and SO4 additions but also for dropping the RA. Let's say by the time you add enough to get your RA down to where you need it, you already have enough or borderline too much Ca or SO4. That's when you'd think about leaving it out of the boil.
When you add a salt to the mash specifically, it's foremost about getting your pH correct.
You can even go a step further by crafting additions that ONLY go into the boil and not in the mash but it starts getting really complicated. Just for example, what you if you need to get a ton of Ca and SO4 into the beer but your RA is already low enough for the mash? Right, gypsum into the boil only.
When you add a salt to the mash specifically, it's foremost about getting your pH correct.
You can even go a step further by crafting additions that ONLY go into the boil and not in the mash but it starts getting really complicated. Just for example, what you if you need to get a ton of Ca and SO4 into the beer but your RA is already low enough for the mash? Right, gypsum into the boil only.
-TH-
Well-Known Member
You can even go a step further by crafting additions that ONLY go into the boil and not in the mash but it starts getting really complicated. Just for example, what you if you need to get a ton of Ca and SO4 into the beer but your RA is already low enough for the mash? Right, gypsum into the boil only.
Hmmm...I sense a revision coming on.
-TH-
Well-Known Member
Hey Kal, I just noticed your little notes below your sulfate about 50-150 being normally bitter and up to 350 being really bitter. How's that range working for you?
I forget if I have read about that in Palmer or not.
Yep, its in Palmer's book:
Sulfate (SO4-2)
Molecular Weight = 96.0
Equivalent Weight = 48.0
Brewing Range = 50-150 ppm for normally bitter beers, 150-350 ppm for very bitter beers
The sulfate ion also combines with Ca and Mg to contribute to permanent hardness. It accentuates hop bitterness, making the bitterness seem drier, more crisp. At concentrations over 400 ppm however, the resulting bitterness can become astringent and unpleasant, and at concentrations over 750 ppm, it can cause diarrhea. Sulfate is only weakly alkaline and does not contribute to the overall alkalinity of water.
It's something I've thought about asking you about but I wonder if the added complexity is worth the occasional use. One idea I had was to have two worksheets that are basically the same but one would be "basic" and one would be "advanced". Basic might be like the version previous to adding the radio buttons. Advanced might be having this explicit control over exactly how much salt you add to the mash and boil. In other words, the boil addition would be a field instead of a checkbox that drives calculation based on the boil entry. I personally, based on my water and the styles I brew, haven't had to do a boil/no mash type addition but after running many profiles from fellow HBT'ers, I can see the utility of it in some cases.
I think as you start looking at ultimate functionality, you start understanding why Palmer's sheet is a clusterF at first glance ;-)
I think as you start looking at ultimate functionality, you start understanding why Palmer's sheet is a clusterF at first glance ;-)
-TH-
Well-Known Member
It's something I've thought about asking you about but I wonder if the added complexity is worth the occasional use. One idea I had was to have two worksheets that are basically the same but one would be "basic" and one would be "advanced". Basic might be like the version previous to adding the radio buttons. Advanced might be having this explicit control over exactly how much salt you add to the mash and boil. In other words, the boil addition would be a field instead of a checkbox that drives calculation based on the boil entry. I personally, based on my water and the styles I brew, haven't had to do a boil/no mash type addition but after running many profiles from fellow HBT'ers, I can see the utility of it in some cases.
I think as you start looking at ultimate functionality, you start understanding why Palmer's sheet is a clusterF at first glance ;-)
I agree totally. Maybe I'll tinker with it a little bit to see if I can come up with a way that doesn't make things more complicated; although as it is right now one of the most common questions with the spreadsheet is when to include or not include salts in the boil.
right now one of the most common questions with the spreadsheet is when to include or not include salts in the boil.
Just so I get this straight. When you are referring to the "boil", that is the sparge water addition right? If I am no-sparge brewing, there is no need to actually add anything to the boil kettle is there? In other words, everything goes into the mash? I know this has been answered before, but it wasn't clear then either
-TH-
Well-Known Member
Just so I get this straight. When you are referring to the "boil", that is the sparge water addition right? If I am no-sparge brewing, there is no need to actually add anything to the boil kettle is there? In other words, everything goes into the mash? I know this has been answered before, but it wasn't clear then either. Semantics? Boil=Sparge?
You are correct. "Sparge" water salt additions are added to the "boil" (which is why people refer to it either way, and hence the confusion).
Thanks for the quick response and explanation. And thanks for the spreadsheet! I'm sure I'll have more questions if/when you revise it. I'm starting to get a handle on mineral additions for mash, but the flavor issue seems to be a moving target.
-TH-
Well-Known Member
I do this already by typing the value directly into the 'Sparge Additions (add to boil)' row. I'm not sure you need to revise the spreadsheet.
Yeah, that might be the best way to go.
Hmm, over-write the calculated sparge/boil addition huh. Face palm!
That works but the downside is that you have to remember you did that if you keep modifying the same file from batch to batch. I personally don't have any problem with modifying my local copy to clear out the formulas from those fields. I'll probably change the bg color to white so I remember that it's a user input field at that point.
Carry on, nothing to see here.
That works but the downside is that you have to remember you did that if you keep modifying the same file from batch to batch. I personally don't have any problem with modifying my local copy to clear out the formulas from those fields. I'll probably change the bg color to white so I remember that it's a user input field at that point.
Carry on, nothing to see here.
kal
Well-Known Member
Ok, I see now. I also don't see why I'd ever add chalk to the boil. Good point. I see now why you're given the option of splitting mash from boil. Makes sense to me!The two salts I regularly leave out of the boil are Chalk and Baking Soda. They are typically only good for raising RA for the mash pH. Also, if you need to use Gypsum and others to drastically lower RA, you'll likely get all the SO4 you'd need out of the mash addition so you wouldn't want to layer more on top for the boil.
I frankly can't remember where I read it either... I think it was in Palmer's book. (edit: oops - you guys already mentioned it!). I just cut and pasted it in there to give me some idea of what to aim for...Hey Kal, I just noticed your little notes below your sulfate about 50-150 being normally bitter and up to 350 being really bitter. How's that range working for you?
I forget if I have read about that in Palmer or not.
I probably have a few other notes here and there I added based on information that I thought would be useful to me.
How well does it work? Good question! I don't know!
I made 11 gallons of Munich Helles today (4.5 SRM) using basically my second set of numbers from above:
Starting Water (ppm):
Ca: 8.9
Mg: 2.2
Na: 16
Cl: 6.1
SO4: 27
CaCO3: 32
Mash / Sparge Vol (gal): 7.3 / 9.4
Dilution Rate: 0%
Adjustments (grams) Mash / Boil Kettle:
CaCO3: 0 / 0
CaSO4: 1 / 1.3
CaCl2: 5 / 6.4
MgSO4: 3 / 3.9
NaHCO3: 0 / 0
NaCl: 0 / 0
HCL Acid: 0 / 0
Lactic Acid: 0 / 0
Mash Water / Total water (ppm):
Ca: 66 / 66
Mg: 12 / 12
Na: 16 / 16
Cl: 93 / 93
SO4: 90 / 90
CaCO3: 32 / 32
RA (mash only): -23 (3 to 8 SRM)
Cl to SO4 (total water): 1.04 (Balanced)
We'll see how it turns out....
Funny enough, I've done 5 batches now from 4-15 SRM and while the RA numbers I come up with should lower my mash pH far enough to get reasonably close to 5.2, for the last two really light beers (last one was a Helles at 4 SRM) I had to end up using a little bit of lactic acid in the mash. In the case of the Helles today noted above, I used 3ml of 88% Lactic Acid in 7.3 gallons of mash to get the pH down from 5.66 to 5.33.
It would seem that (at least for me) if the spreadsheet gives an SRM range then I need to target the upper end of the range (ex: the numbers above say it's good for a 3-8 SRM beer but for me I'll only get pH into range for the higher end, or 8 SRM). For very light coloured beers it's meant using lactic acid a bit. The darker beers (10-15 SRM) that I made previously seem to get a mash pH to low enough (5.3-5.4) that I don't bother. Having a pH meter is a must!
Kal
I'm probably going to splurge and drop $70 on a meter before my next batch because I'm less confident that the strips are good enough. Well, actually it's because I'm a gadget geek.
kal
Well-Known Member
I'm probably going to splurge and drop $70 on a meter before my next batch because I'm less confident that the strips are good enough. Well, actually it's because I'm a gadget geek.
For me it's more the number of times I take readings. I'm still new at water measurements/pH stuff so when I brewed my last batch today, I took around 20 pH readings just to learn what's going on. That's a lot of strips! ($$$$!)
Once I get good at this and start to repeat batches, I likely won't bother as much but for now, readings go more or less like this:
1. Measure initial water pH
2. Dough in, measure pH.
3. Add salts, measure pH so that I see how salts affect mash pH.
4. Hmmm, pH still too high, so add a little bit of lactic acid. Measure pH. Repeat until we're around 5.2-5.4.
5. Mash for XX minutes. Measure pH at end of mash just out of curiosity.
6. Acidify strike water if it's a lighter beer. Measure pH, add lactic, repeat to get strike water down to 6 pH.
7. Sparge. Measure at 5 gallon point, 8, 10, 12, 14. We want to make sure pH stays below 5.6-6.0 range (5.8 preferred) to avoid excess tannin extraction.
8. Measure pH of collected wort. Add salts. Measure pH. 5.5 is considered "perfect".
9. Boil. Measure pH. Should be around 5.2.
10. Ferment. Measure pH of finished product if curious.
Kal
garretto
Well-Known Member
You have a situation where there's no harm in adding any of those salts to the boil since they are flavor affecting. Let's say you were adding a bunch of gypsum to the mash for Ca and SO4 additions but also for dropping the RA. Let's say by the time you add enough to get your RA down to where you need it, you already have enough or borderline too much Ca or SO4. That's when you'd think about leaving it out of the boil.
When you add a salt to the mash specifically, it's foremost about getting your pH correct.
You can even go a step further by crafting additions that ONLY go into the boil and not in the mash but it starts getting really complicated. Just for example, what you if you need to get a ton of Ca and SO4 into the beer but your RA is already low enough for the mash? Right, gypsum into the boil only.
Awwww alright, the lightbulb just lit up. That makes a lot more sense. Great job explaining.
And thanks -TH- and Kal for the sulfate brush up.
I hope all your batches come out great, Kal.
Hmm, over-write the calculated sparge/boil addition huh. Face palm!
That works but the downside is that you have to remember you did that if you keep modifying the same file from batch to batch. I personally don't have any problem with modifying my local copy to clear out the formulas from those fields. I'll probably change the bg color to white so I remember that it's a user input field at that point.
Carry on, nothing to see here.
I start with the original spreadsheet and after making modifications I save it with the style and date brewed in the title. That way I always start with the original spreadsheet.
I just got my water report today and I have to say there is some great information here. Thanks everyone.
I am confused on the value to use for sparge water. I am assuming this is the actual amount used for sparge and not the collected amount? In other words the total water for mash and sparge combined will be greater than the boil volume? *edit* I just watched the video (wouldnt work at first) and that seemed to anwer this.
I noticed earlier that salts are added to the mash (I would have tried the strike water). So, boil additions will go into the kettle after sparging right?
thanks for the help and good info.
I am confused on the value to use for sparge water. I am assuming this is the actual amount used for sparge and not the collected amount? In other words the total water for mash and sparge combined will be greater than the boil volume? *edit* I just watched the video (wouldnt work at first) and that seemed to anwer this.
I noticed earlier that salts are added to the mash (I would have tried the strike water). So, boil additions will go into the kettle after sparging right?
thanks for the help and good info.
Can I hijack this thread? Or shall I start a new one?
Just got back my water report from Ward Labs and this is what I got:
Starting Water (ppm):
Ca: 60
Mg: 39
Na: 24
Cl: 21
SO4: 15
HCO3: 333
Total Hardness CaCO3: 313
Total Alkalinity, CaCO3: 273
pH: 7.8
RA (mash only): 207 (22 to 27 SRM)
Cl to SO4 (total water): 1.40 (Malty)
Looks good for darker beer, which I like, but I also like IPA's and such. I tried the dilution ratio but I had to get about 90% to get the mash RA where it needs to be. Any suggestions??
thanks in advance
Aaron
Just got back my water report from Ward Labs and this is what I got:
Starting Water (ppm):
Ca: 60
Mg: 39
Na: 24
Cl: 21
SO4: 15
HCO3: 333
Total Hardness CaCO3: 313
Total Alkalinity, CaCO3: 273
pH: 7.8
RA (mash only): 207 (22 to 27 SRM)
Cl to SO4 (total water): 1.40 (Malty)
Looks good for darker beer, which I like, but I also like IPA's and such. I tried the dilution ratio but I had to get about 90% to get the mash RA where it needs to be. Any suggestions??
thanks in advance
Aaron
First off, thanks for great videos Bobby and a great spreadsheet TH!
After getting an email back from my water company, it seems the only numbers I am missing to properly use this chart is Chloride and Sulfate which seem to be very important.
Their reasoning was "I do not expect to detect Sulfate and Chloride would be bound with Calcium, Sodium, and other salts." I guess I can't make them test for it if they haven't but they seem pretty cool to answer any question so I was wondering if anyone knew a different way to ask for those numbers? I thought they would be pretty common for a water company to know.
I will probably just order Ward labs if this doesn't work out but figured free was worth a shot first.
After getting an email back from my water company, it seems the only numbers I am missing to properly use this chart is Chloride and Sulfate which seem to be very important.
Their reasoning was "I do not expect to detect Sulfate and Chloride would be bound with Calcium, Sodium, and other salts." I guess I can't make them test for it if they haven't but they seem pretty cool to answer any question so I was wondering if anyone knew a different way to ask for those numbers? I thought they would be pretty common for a water company to know.
I will probably just order Ward labs if this doesn't work out but figured free was worth a shot first.
Can I hijack this thread? Or shall I start a new one?
Just got back my water report from Ward Labs and this is what I got:
Starting Water (ppm):
Ca: 60
Mg: 39
Na: 24
Cl: 21
SO4: 15
HCO3: 333
Total Hardness CaCO3: 313
Total Alkalinity, CaCO3: 273
pH: 7.8
RA (mash only): 207 (22 to 27 SRM)
Cl to SO4 (total water): 1.40 (Malty)
Looks good for darker beer, which I like, but I also like IPA's and such. I tried the dilution ratio but I had to get about 90% to get the mash RA where it needs to be. Any suggestions??
thanks in advance
Aaron
Did you watch the videos by Bobby M. That sure answered a lot of my questions. You will definately need some salt aditions to get to a light colored beer. The spreadsheet recommended is great! I just plugged in some numbers for salts until I got a reasonable result. I am very excited to try brewing a lighter beer now. They never turned out very well before.
Good Luck
Did you watch the videos by Bobby M. That sure answered a lot of my questions. You will definately need some salt aditions to get to a light colored beer. The spreadsheet recommended is great! I just plugged in some numbers for salts until I got a reasonable result. I am very excited to try brewing a lighter beer now. They never turned out very well before.
Good Luck
Yes I have watched them, in fact i watched them again. It seems that I need to dilute my water first then add back, without having to add a bunch of salts. I'll go through it again later today and post what I think needs to happen and see what the brain trust has to say about that.

Aaron
Yes I have watched them, in fact i watched them again. It seems that I need to dilute my water first then add back, without having to add a bunch of salts. I'll go through it again later today and post what I think needs to happen and see what the brain trust has to say about that.
Aaron
If you are interested here is my water profile:
Starting Water (ppm):
Ca: 31.9
Mg: 13.9
Na: 2.6
Cl: 4.1
SO4: 14.4
CaCO3: 123
RA (mash only): 92 (13 to 18 SRM)
Cl to SO4 (total water): 0.28 (Very Bitter)
Here are the adjustements made for a 5.2 SRM wheat. This is my first attempt so if anyone sees a problem I would appreciate any advice.
Starting Water (ppm):
Ca: 31.9
Mg: 13.9
Na: 2.6
Cl: 4.1
SO4: 14.4
CaCO3: 123
Mash / Sparge Vol (gal): 6.25 / 9.5
Dilution Rate: 0%
Adjustments (grams) Mash / Boil Kettle:
CaCO3: 0 / 0
CaSO4: 7 / 0
CaCl2: 2 / 3.04
MgSO4: 2 / 0
NaHCO3: 0 / 0
NaCl: 5 / 0
HCL Acid: 0 / 0
Lactic Acid: 2 / 0
Mash Water / Total water (ppm):
Ca: 122 / 82
Mg: 22 / 17
Na: 86 / 36
Cl: 173 / 96
SO4: 212 / 93
CaCO3: 123 / 123
RA (mash only): -27 (3 to 8 SRM)
Cl to SO4 (total water): 1.03 (Balanced)
I thought I may have to dilute with RO water. But it seems I was able to get where I wanted with salts.
Inhiding,
You can do an IPA or Pale Ale with a 50% distilled dilution assuming about 4 gallons mash, 4 gallons sparge, change your numbers as necessary. This is a great example of why you'd put a bunch of salts into the mash and then hold them back from the boil.
If you primarily brew paler beers, you might want to seriously consider getting an RO system. It will pay for itself after 100 gallons of storebought RO/Distilled.
Starting Water (ppm):
Ca: 60
Mg: 39
Na: 24
Cl: 21
SO4: 15
CaCO3: 273
Mash / Sparge Vol (gal): 4 / 4
Dilution Rate: 50%
Adjustments (grams) Mash / Boil Kettle:
CaCO3: 0 / 0
CaSO4: 8 / 0
CaCl2: 3 / 0
MgSO4: 0.5 / 0
Mash Water / Total water (ppm):
Ca: 204 / 117
Mg: 23 / 21
Na: 12 / 12
Cl: 106 / 58
SO4: 315 / 161
CaCO3: 137 / 137
RA (mash only): -22 (3 to 8 SRM)
Cl to SO4 (total water): 0.36 (Very Bitter)
You can do an IPA or Pale Ale with a 50% distilled dilution assuming about 4 gallons mash, 4 gallons sparge, change your numbers as necessary. This is a great example of why you'd put a bunch of salts into the mash and then hold them back from the boil.
If you primarily brew paler beers, you might want to seriously consider getting an RO system. It will pay for itself after 100 gallons of storebought RO/Distilled.
Starting Water (ppm):
Ca: 60
Mg: 39
Na: 24
Cl: 21
SO4: 15
CaCO3: 273
Mash / Sparge Vol (gal): 4 / 4
Dilution Rate: 50%
Adjustments (grams) Mash / Boil Kettle:
CaCO3: 0 / 0
CaSO4: 8 / 0
CaCl2: 3 / 0
MgSO4: 0.5 / 0
Mash Water / Total water (ppm):
Ca: 204 / 117
Mg: 23 / 21
Na: 12 / 12
Cl: 106 / 58
SO4: 315 / 161
CaCO3: 137 / 137
RA (mash only): -22 (3 to 8 SRM)
Cl to SO4 (total water): 0.36 (Very Bitter)
Hey, I want to brew a Maris Otter/Fugles SMaSH this weekend. I'd like to try for around 20-25 IBU. Have no idea what SRM would be, kinda thinking around 6-8. I'm doing a no-sparge process with appr. 8 gals. volume. Can someone look at these additions and see if I'm at least in the ballpark. I'm kinda concerned about the Mg, cuz' I know that yeast love Mg. Thanks
Starting water (ppm):
Ca: 8
Mg: 2
Na: 25
Cl: 15
SO4: 11
CaCO3: 20
Mash / Sparge Vol (gal):8/0
Dilution Rate:0%Adjustments (grams) Mash / Boil
Kettle:CaCO3: 2.25/0
CaSO4: 0/0
CaCl2: 2/0
MgSO4: 3/0
NaHCO3: 0/0
NaCl: 0/0
HCL Acid: 0/0
Lactic Acid: 0/0
Mash Water / Total water (ppm):
Ca: 56/56
Mg: 11/11
Na: 25/25
Cl: 47/47
SO4: 50/50
CaCO3: 57/57
RA (mash only): 10 (6 to 11 SRM)
Cl to SO4 (total water): 0.94 (Balanced)
Starting water (ppm):
Ca: 8
Mg: 2
Na: 25
Cl: 15
SO4: 11
CaCO3: 20
Mash / Sparge Vol (gal):8/0
Dilution Rate:0%Adjustments (grams) Mash / Boil
Kettle:CaCO3: 2.25/0
CaSO4: 0/0
CaCl2: 2/0
MgSO4: 3/0
NaHCO3: 0/0
NaCl: 0/0
HCL Acid: 0/0
Lactic Acid: 0/0
Mash Water / Total water (ppm):
Ca: 56/56
Mg: 11/11
Na: 25/25
Cl: 47/47
SO4: 50/50
CaCO3: 57/57
RA (mash only): 10 (6 to 11 SRM)
Cl to SO4 (total water): 0.94 (Balanced)
It looks good to me. I wouldn't change anything. 10lbs of maris should give you something like 1.050 with an SRM of 7.
Thanks Bobby. I changed it a bit. Went with 2.25 CaCO3, 3.25 CaCL2,and 4.5 MgSO4. Came out to RA of -1 and .97 CL/SO4 ratio. It's mashing now. Beersmith says 1.049 OG and and SRM of 5.1. Nothing to do now but wait to see how it turns out.
My water is very low mineral and shows very malty unadjusted. We brewed an extract IPA recipe at my house and it was flat tasting compared to the same recipe at a buddy's house. So I was wondering, for extract, if I made the adjustment only to the chloride to sulfate ratio like this:
Starting Water (ppm):
Ca: 2
Mg: 1.1
Na: 6.5
Cl: 8.4
SO4: 4.1
CaCO3: 11
Mash / Sparge Vol (gal): 7 / 0
Dilution Rate: 0%
Adjustments (grams) Mash / Boil Kettle:
CaCO3: 0 / 0
CaSO4: 0 / 0
CaCl2: 0 / 0
MgSO4: 0 / 0.5
NaHCO3: 0 / 0
NaCl: 0 / 0
HCL Acid: 0 / 0
Lactic Acid: 0 / 0
Mash Water / Total water (ppm):
Ca: 2 / 2
Mg: 3 / 3
Na: 7 / 7
Cl: 8 / 8
SO4: 11 / 11
CaCO3: 11 / 11
RA (mash only): 8 (6 to 11 SRM)
Cl to SO4 (total water): 0.73 (Bitter)
So I guess the question here is does the chloride to sulfate ratio pertain to the boil and not the mash, to just the mash, or both? (This is just a general question if the ratio does something in the mash or boil)
The next question is only if I should make the adjustment. Would I use the volume of the boil in the spreadsheet or the finished volume of the wort if I do a partial boil? I'm guessing just the boil volume, but not positive.
TIA
Starting Water (ppm):
Ca: 2
Mg: 1.1
Na: 6.5
Cl: 8.4
SO4: 4.1
CaCO3: 11
Mash / Sparge Vol (gal): 7 / 0
Dilution Rate: 0%
Adjustments (grams) Mash / Boil Kettle:
CaCO3: 0 / 0
CaSO4: 0 / 0
CaCl2: 0 / 0
MgSO4: 0 / 0.5
NaHCO3: 0 / 0
NaCl: 0 / 0
HCL Acid: 0 / 0
Lactic Acid: 0 / 0
Mash Water / Total water (ppm):
Ca: 2 / 2
Mg: 3 / 3
Na: 7 / 7
Cl: 8 / 8
SO4: 11 / 11
CaCO3: 11 / 11
RA (mash only): 8 (6 to 11 SRM)
Cl to SO4 (total water): 0.73 (Bitter)
So I guess the question here is does the chloride to sulfate ratio pertain to the boil and not the mash, to just the mash, or both? (This is just a general question if the ratio does something in the mash or boil)
The next question is only if I should make the adjustment. Would I use the volume of the boil in the spreadsheet or the finished volume of the wort if I do a partial boil? I'm guessing just the boil volume, but not positive.
TIA
kal
Well-Known Member
My water is very low mineral and shows very malty unadjusted.
Ca: 2
Mg: 1.1
Na: 6.5
Cl: 8.4
SO4: 4.1
CaCO3: 11
Wow. You've got nearly RO water there coming out of your tap! I'm jealous!
Mash Water / Total water (ppm):
Ca: 2 / 2
Mg: 3 / 3
Na: 7 / 7
Cl: 8 / 8
SO4: 11 / 11
CaCO3: 11 / 11
RA (mash only): 8 (6 to 11 SRM)
Cl to SO4 (total water): 0.73 (Bitter)
So I guess the question here is does the chloride to sulfate ratio pertain to the boil and not the mash, to just the mash, or both?
The Cl:S04 ratio applies to the total water as it applies to the taste. So both mash and boil.
I would go higher on all your numbers here. You should try and at least hit the minimums recommended (CA at least 50, Mg at least 10) in the spreadsheet and for an IPA I would try and target Moser's ideal pale ale which is higher around all over. While your Cl:S04 ratio may be ok (I'd probably go even more to the bitter side) the amounts of Cl and S04 also matter. They're too low if you ask me. Look at Moser's numbers. Target that.
Kal
Oops, missed a critical word in there. This would be an extract batch. I've used the spreadsheet successfully for my all grain. We do a big group extract boil of 10 gallons for 20 gallons of wort. I'll edit it above.
Similar threads
- Replies
- 9
- Views
- 762
- Article
- Replies
- 12
- Views
- 2K

