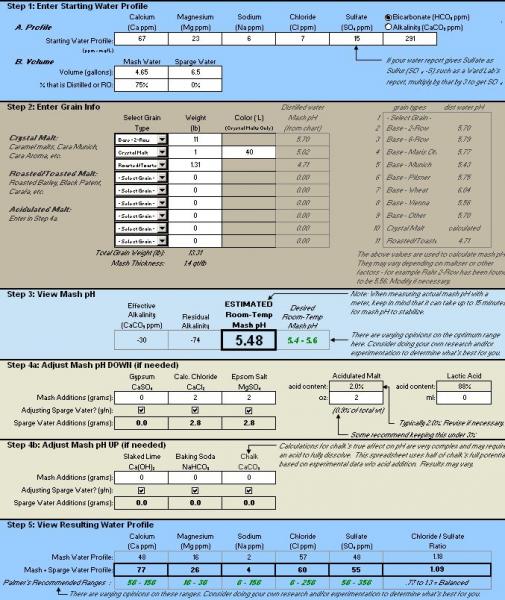
HI,
I was really hoping someone could take a look at my results with palmers Mash RA spreadsheet. I think I got it right but I wanted to make sure.
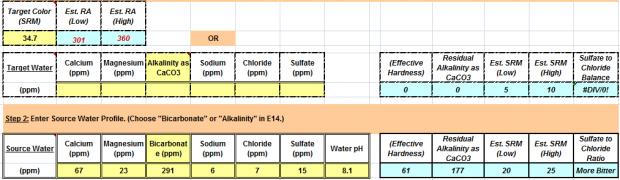
Source Water + SRM:
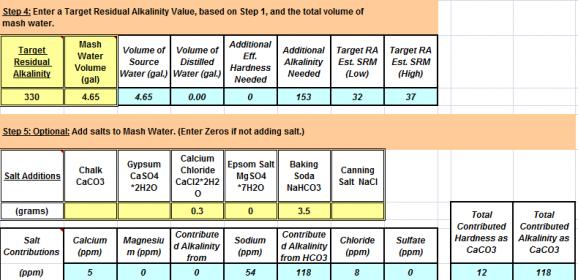
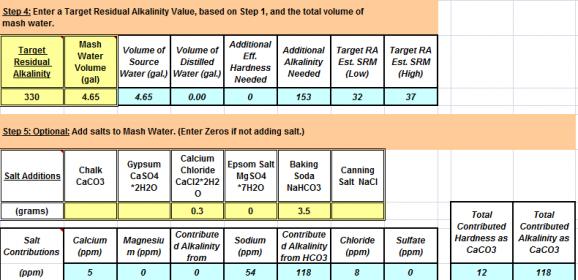
Mash Water + Salt additions:
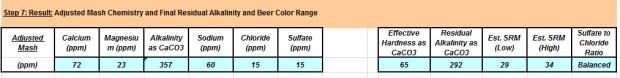
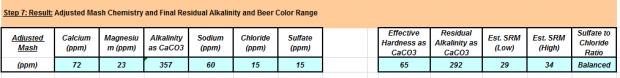
Water Results:
Anyone see any issue with my plan? Or have any thoughts?
Thank you so much for looking at this!

I was really hoping someone could take a look at my results with palmers Mash RA spreadsheet. I think I got it right but I wanted to make sure.
Source Water + SRM:
Mash Water + Salt additions:
Water Results:
Anyone see any issue with my plan? Or have any thoughts?
Thank you so much for looking at this!