Mer-man
Well-Known Member
I just moved to Copenhagen and am grappling with the super high alkalinity of the tapwater. The water reports here are insanely detailed and up to date, and according to Bru'n Water my ions balance near perfectly.
But I'm looking at (edit: 380ppm I think and not 549, but still!) 549ppm bicarbonate here. Naturally I want to remove the vast majority of the alkalinity so I can brew Pale Ales and such. The water in Singapore was basically a blank slate but here it is . . . robust. I have attached a screenshot of the basic water parameters.
Sadly, you can't buy RO water by the jug here and I rent, so an RO system will have to wait. I have lactic acid (sold strangely at 80% from maltbazaren.dk) but according to Bru'n, I would need a helluva lot.
So I am looking at boiling 20L or so for 30 minutes and adding the following to that water:
2g CaSO4
2.5g CaCl
2ml 80% lactic acid
I couldn't work this out with Bru'n, but brewersfriend's boil calculator predicted that this should drop nearly all the bicarbonate out of the water. I would then blend the water back 50/50 with my tap water.
And according to Bru'n, I would still need to add a few ml of acid to kill off the remaining bicarbonate to get my mash pH to 5.4. But the ultimate acid use is a fraction of the non-boiling approach.
Are there huge flaws to my plan or is it doable? And should I add the acid before I boil that 20L (my guess is yes)?
Also, is there any way to reduce Chloride without dilution? The water here is way high in it.
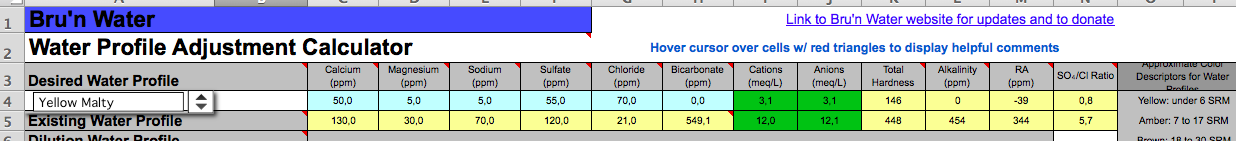
But I'm looking at (edit: 380ppm I think and not 549, but still!) 549ppm bicarbonate here. Naturally I want to remove the vast majority of the alkalinity so I can brew Pale Ales and such. The water in Singapore was basically a blank slate but here it is . . . robust. I have attached a screenshot of the basic water parameters.
Sadly, you can't buy RO water by the jug here and I rent, so an RO system will have to wait. I have lactic acid (sold strangely at 80% from maltbazaren.dk) but according to Bru'n, I would need a helluva lot.
So I am looking at boiling 20L or so for 30 minutes and adding the following to that water:
2g CaSO4
2.5g CaCl
2ml 80% lactic acid
I couldn't work this out with Bru'n, but brewersfriend's boil calculator predicted that this should drop nearly all the bicarbonate out of the water. I would then blend the water back 50/50 with my tap water.
And according to Bru'n, I would still need to add a few ml of acid to kill off the remaining bicarbonate to get my mash pH to 5.4. But the ultimate acid use is a fraction of the non-boiling approach.
Are there huge flaws to my plan or is it doable? And should I add the acid before I boil that 20L (my guess is yes)?
Also, is there any way to reduce Chloride without dilution? The water here is way high in it.

Last edited:


