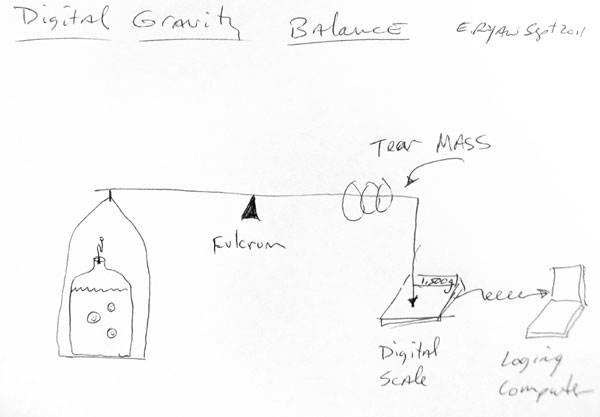
I ferment with a balloon as my airlock. The fun advantage of this is it allows you to roughly measure the rate of fermentation. If it takes roughly twenty seconds to fill a ~75ml balloon with CO2, then you can crunch the numbers and figure out that roughly 93.3 Quintilian (9.33x10^19) molecules of CO2* are being produced every second.
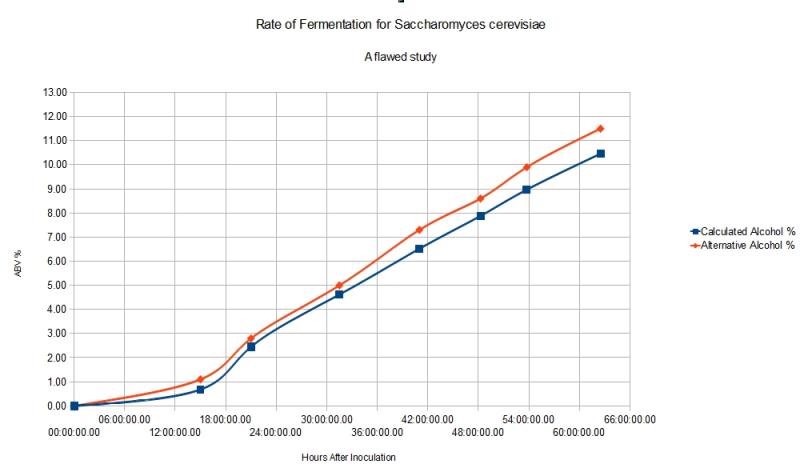
We also know that 2 molecules of CO2* are made from every sugar molecule. If the population size is known, you can then calculate the rate of fermentation per cell (since yeast are unicellular organisms). The hard part is counting them all.
If you also know the maximum alcohol tolerance of the specific yeast you are using, then you can estimate the appropriate amount of sugar to add to achieve a desired result, be it dry or sweet. This would be useful for recipies you've not yet tried before. Tracking the rate of fermentation at several hour intervals also offers insight into the alcohol tolerance of certain yeasts, assuming abundant sugar and appropriate temperature.
Footnotes:
* - and ethanol
EDIT: DATA POSTED! Click to go to post
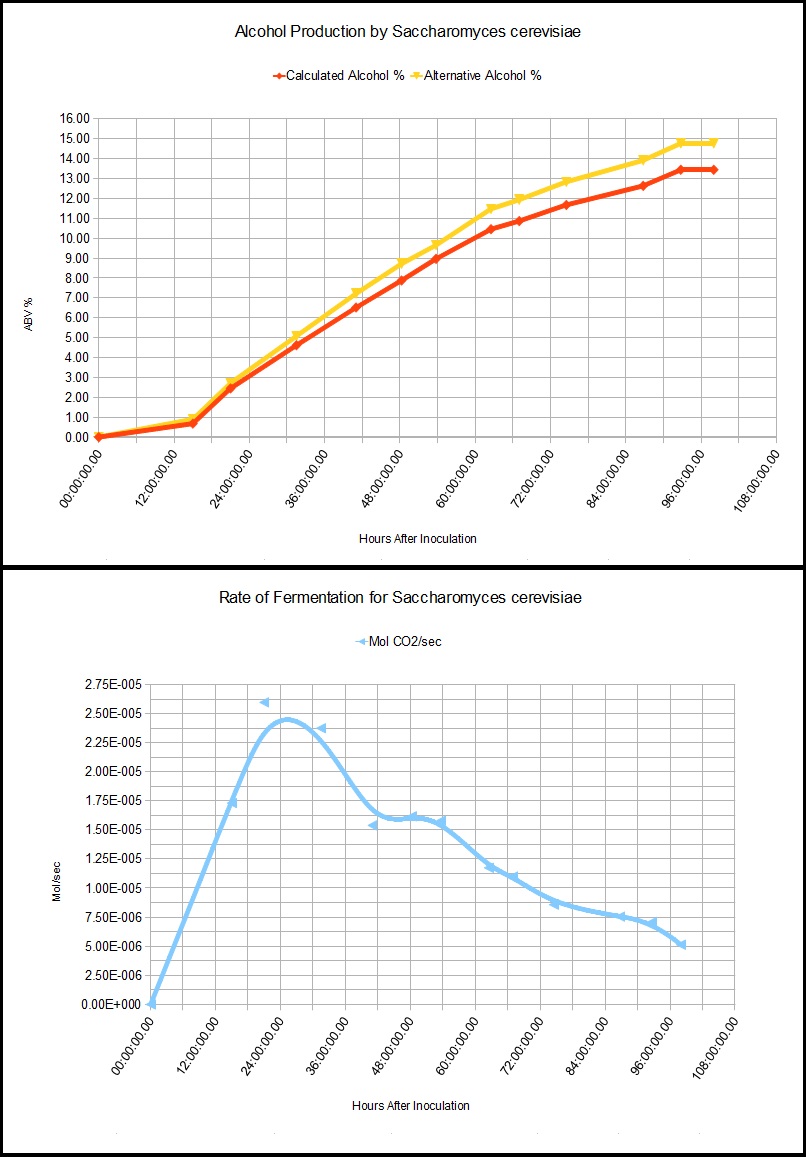
We also know that 2 molecules of CO2* are made from every sugar molecule. If the population size is known, you can then calculate the rate of fermentation per cell (since yeast are unicellular organisms). The hard part is counting them all.
If you also know the maximum alcohol tolerance of the specific yeast you are using, then you can estimate the appropriate amount of sugar to add to achieve a desired result, be it dry or sweet. This would be useful for recipies you've not yet tried before. Tracking the rate of fermentation at several hour intervals also offers insight into the alcohol tolerance of certain yeasts, assuming abundant sugar and appropriate temperature.
Footnotes:
* - and ethanol
EDIT: DATA POSTED! Click to go to post