There are only a few styles that have shown a need for less hardness than the typical 125 ppm as CaCO3 that 50 ppm of Ca provides.
No style
needs it. You can brew a Pils with harder water if you want to. It is just likely that you will like the beer better if you reduce it. And this extends beyond Pils certainly to the rest of the lager family. Again it really is a matter of personal preference but the trend is towards brewing with lower levels of hardness as the consumers like the beer better.
AJ happens to be a proponent and aficiando of those styles, but my preferences lay in ales.
Then we should recognize that the hardness level depends on what one's personal preferences are and allow that there may indeed be cases where RO is advantageous.
But there are definite consequences from brewing with low Ca water.
Better beer unless you like 'harder' beers and of course some do.
More beerstone potential,
Reminds me of a paper about formation of calcium oxalate. The author said that it could be explained 25% by the thermodynamics, 25% by ionic strength considerations (which I'd consider part of the thermodynamics but that's besides the point and 50% by factors which are not understood. I'm paraphrasing here and don't really remember the exact numbers. The implication that low calcium will increase the probability of its deposition is quite interesting when the medical advice given to the kidney stone fraternity is considered. Some doctors say take a calcium supplement which supports the notion that increased calcium drops it (in the gut vs. the kidneys) but some doctors say drink softened water which supports the idea that no calcium - no calcium oxalate. We wonder if our bretheren in the Pacific Northwest with their RO like water suffer higher incidence of beer stone and I wonder if I suffer it more than others in my area that don't use RO water (and keep it soft). We also wonder how many home brewers have ever experienced it under any condition. Chime in if you have.
Anyway beer stone has never been a problem for me because I take steps to prevent its accumulation with both kegs and fermentors whether I need to or not. Maybe I do need to because I use soft water. Maybe I don't.
This has never been a problem in my experience.
reduced clarification performance.
Never been a problem either. In fact I found that when I went to softer water and used sauermalz to control the pH that my beers dropped clear faster. At least the lagers did. With a dusty yeast it doesn't seem to make much difference but I've never measured it on Kölsch. That's going to be hazy unless you filter it.
All of these maladies can be overcome through modified brewing practices and procedures like cleaning equipment more often, pitching more yeast, and using mechanical filtration. If you aren't willing to take those extra steps, then low Ca brewing may not be ideal for brewing.
I have never been aware that I am doing anything other than using good brewing practices. I clean my equipment, I make starters, I oxygenate as I pitch, I control fermentation temperature. I do this whether I am making a soft water beer or not.
In the case of pretty much all beers with more robust flavor, including adequate Ca concentration in the brewing water is no detriment at all. Using RO water and building water is no guarantee for brewing success over using this moderately hard Tally water.
No, there is no guarantee, but there is a very good chance that appreciable improvement could be realized. I encourage readers to try it and see for themselves. To quote an old Charlie Chan movie: "Mind like parachute. Function best when open."
I have had the fortune of my beers scoring as high as 42 at the FL state fair contest, so I'd say someone else could do it too.
Maybe they could have scored 45.
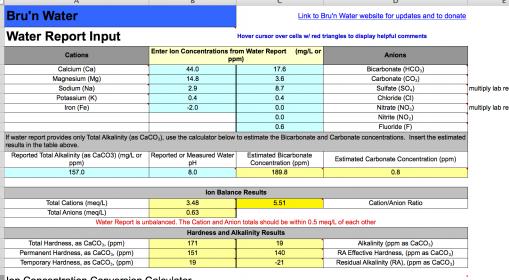
When a brewer has a tool like Bru'n Water, figuring out an acid addition is a 10 second ordeal that will probably bring them to tears, but you just have to buck up and do it for the beer!
When a brewer has a tool like an RO source it is a 1 second ordeal. Want half the alkalinity? Dilute 1:1. Don't want to mess with alkalinity at all? Use straight RO. You can use a spreadsheet to calculate the mineral supplements of course.
Fortunately, in dealing with that total PITA that a brewer has to go through in acidifying their water using the Bru'n Water tool, they also have the ability to moderate that acid addition and actually dial in the appropriate alkalinity needed for their current brew.
Actually, the computations are pretty complicated but fortunately the error induced by not accounting for the effects of CO2 leaving the solution are small e.g. the case in point if you wanted to knock off 100 ppm of the alkalinity you could simply add 2 mmol/L phosphoric acid and arrive, after agitation, at alkalinity 59 - only 2 ppm off. Note that the complexities from the polyprotic nature of phosphoric acid don't even have to be accounted for.
I'm starting to think that a required or appropriate alkalinity for a brew is an notion whose time has gone (Martin - this thought germinated while thinking about the book). What is required is the amount of acid or base needed to set mash pH correctly. This could be put into the water or it could be put into the mash based on a test mash.
If you start with RO, you had better have the minerals necessary to add back alkalinity for some styles.
Yes. Not a big deal to me as I start from RO water anyway thus giving me total flexibility WRT to what goes into the mash tun (subject, of course, to the laws of physics). I would never, even if I used tap water, try to increase the alkalinity of the water because to do so with calcium bicarbonate, is so difficult and I don't like sodium in my beers. I would use pickling lime in the mash tun and I expect you would too.
The selection of acid is an important consideration.
Yes it is and that's one of the reasons I think it is simpler to use RO than remove alkalinity with acid.
In my opinion and experience, no brewer in Tally needs or will really benefit from their own RO system.
In my opinion and experience they very probably will but that's my opinion and experience. Even so I would never make a statement like: I can defintely tell you that you must use RO as it is the only path to good beer. I will say that there is a good chance that it will and that people should try it and see but I've said that before. Guess I don't understand the resistance to the idea.
They already make excellent beer and this is further confirmed by multiple World Beer Cup...]
The brew pub I'm associated with has also taken medals at GABF but the brewer there recognizes that RO will probably give him a better Pils and Kölsch. We haven't installed the system yet (and won't till I get back south) and of course time will tell but at least he has an open mind.