abbysdad2006
Well-Known Member
You're not far off from a SO4/Cl of 2:1. A touch of sulfate is all you would need. But how much depends on the volume of water you're using.
Thanks guys
So you don't touch the Cl any just adjust the SO4?

You're not far off from a SO4/Cl of 2:1. A touch of sulfate is all you would need. But how much depends on the volume of water you're using.
Depends on what profile you're aiming for. But if you're strictly just worried about the SO4/Cl ratio (which honestly - and no offense meant - if you didn't know/understand the scientific symbols (Ca, Mg, etc), I'd say you shouldn't be worried about this ratio too much as it would seem to be more of an advanced topic than the level you are on), then currently you're at about 1.84:1. If you increase the Cl content (without compensating by also adding even more sulfate), that will drive the ratio down, not up. If you're steadfast on hitting 2:1 and not concerned with any other additions, I would suggest calcium chloride, as it will increase your SO4 content as well as calcium, which you have a little room to play with (perhaps aim for 100ppm total Ca).
If you're steadfast on hitting 2:1 and not concerned with any other additions, I would suggest calcium chloride, as it will increase your SO4 content as well as calcium, which you have a little room to play with (Ca can reasonably be in the 100-150ppm range, though I tend to stick to the 100ppm area for most beers).
If I use water from RO refill stations (glacier or primo, etc.) and use a water calculator do I leave all the source water values blank? (Ca, mg, so4, etc.) or does RO not strip everything out? Sorry for stupid question but having serious water issues with three failed pale ales since moving back to SW Texas with its apparently crappy desert water
What advice? RO + CaCl? I've scored in the 40s on a couple of beers made that way. The general advice to measure and control mash pH rather than trying to predict? Same, only more than a couple.
The ph meter is the single best hot side investment I have made (and useful on the cold side as well).
So, you say test the PH of the mash. I've read that testing PH while the source is hot both shortens the life of the PH meter probe, and gives inaccurate readings. What is your experience with that? Also, if you do cool samples down, how?
Hefeweizen: Baseline
Baseline: Add 1 tsp of calcium chloride dihydrate (what your LHBS sells) to each 5 gallons of water treated. Add 2% sauermalz to the grist.
Deviate from the baseline as follows:
Hefeweizen: For soft water beers (i.e Pils, Helles). Use half the baseline amount of calcium chloride and increase the sauermalz to 3% (you can make great Hefe with soft water too).
Porter: For beers that use roast malt (Stout, porter): Skip the sauermalz.
IPA: For very minerally beers (Export, Burton ale): Double the calcium chloride and the gypsum.
Unfortunately, a side effect seems to be that my mash pH (room temp) has been coming in around 0.15 - 0.20 below my target in bru'n water and I'm not sure what might be causing it.

Thanks for the suggestions here regarding the low measured mash pH I've been seeing on my recent batches. I understand the comment that the water calculators are dependent on characteristics of the malt, and that the best way to get accurate results would be to measure the pH of a sample of each malt in DI. Unfortunately, it's hard enough for me to find the time to brew as much as I'd like to, so I can't see myself taking on this task for every malt that each recipe I brew calls for. I could maybe see myself trying it for a few of the most common base malts I use, but it seems like the dark specialty malts may have the most significant impact so i'm not sure whether this would even be useful.
For now, I think I'll look for a couple of other calculators to try to see if they would have gotten me closer to my measured pH than I've been getting with bru'n water. I guess what still seems odd to me is that so many users seem to reliably be within 0.05 pH using this software, and yet I'm coming in consistently 0.15-0.20 low with a wide variety of grain bills so I can't imagine it being one specific malt type that I'm using that the software is having trouble with.
If I wanted to try to "fudge" it with bru'n water, what would be the best way to go about this? It would be easiest if I could do something to the water profile so that I don't have to increase the color of each grain by some arbitrary amount, but even changing my setting from 100% RO to DI only gets me about 0.04 closer to what I'm actually measuring. As a side note, I'm measuring mash pH at room temp with an Omega pH meter that I calibrate while I'm waiting for my sample to cool and which claims an accuracy of +/- 0.01.
This is the best way but it is certainly not practical. I have campaigned (though not that vigorously) to get the maltsters to make the measurements when they are doing their Congress mashes for color but the enthusiasm has not been overwhelming.Thanks for the suggestions here regarding the low measured mash pH I've been seeing on my recent batches. I understand the comment that the water calculators are dependent on characteristics of the malt, and that the best way to get accurate results would be to measure the pH of a sample of each malt in DI.
It is much more practical to do this with a small sample of the grist for each mash. In fact all you have to do with such a test mash is determine how much acid it takes to get it to the desired pH and then scale that to the full mash size.Unfortunately, it's hard enough for me to find the time to brew as much as I'd like to, so I can't see myself taking on this task for every malt that each recipe I brew calls for.
It is actually the base malts that have the major impact simply because they make up the bulk of the mash. The exception is the really dark beers where the specialty malts are relied upon to do what is done with acid in lighter beers.I could maybe see myself trying it for a few of the most common base malts I use, but it seems like the dark specialty malts may have the most significant impact so i'm not sure whether this would even be useful.
Brewers use a wide variety of grains and Bru'n water has models that are more acidic than some and less acidic than others. What the errors turn out to be depends on the match between the models and the actual malts. Sometimes the agreement will be good and sometimes it won't.I guess what still seems odd to me is that so many users seem to reliably be within 0.05 pH using this software,
Indeed if you see a consistent bias then you are right to suspect systematic error which is probably not in the program itself but rather in your application of it. Are you doing the pH measurements correctly? Is there some setting in the program that is not what you think it is? You might try posting one of your interactions with it here and letting other guys who use it run the numbers too to see if they get the same answers....and yet I'm coming in consistently 0.15-0.20 low with a wide variety of grain bills so I can't imagine it being one specific malt type that I'm using that the software is having trouble with.
I think it has already been mentioned here but if the gas gauge in your car always reads 1/4 tank high you quickly learn to pull over for a fill up when the needle approaches 1/4 full. Thus here you would simply add 0.175 to each pH estimate. I would encourage looking further for the source of the error, however.If I wanted to try to "fudge" it with bru'n water, what would be the best way to go about this?
It actually shouldn't make any difference as we assume that RO water is ion free in doing these calculations.It would be easiest if I could do something to the water profile so that I don't have to increase the color of each grain by some arbitrary amount, but even changing my setting from 100% RO to DI only gets me about 0.04 closer to what I'm actually measuring.
But how accurate is it in actuality. If you are calibrating it with the typical NIST traceable operational buffers (±0.02) it isn't accurate to ±0.01. How does it do on the stability test (https://www.homebrewtalk.com/showthread.php?t=302256)?As a side note, I'm measuring mash pH at room temp with an Omega pH meter that I calibrate while I'm waiting for my sample to cool and which claims an accuracy of +/- 0.01.
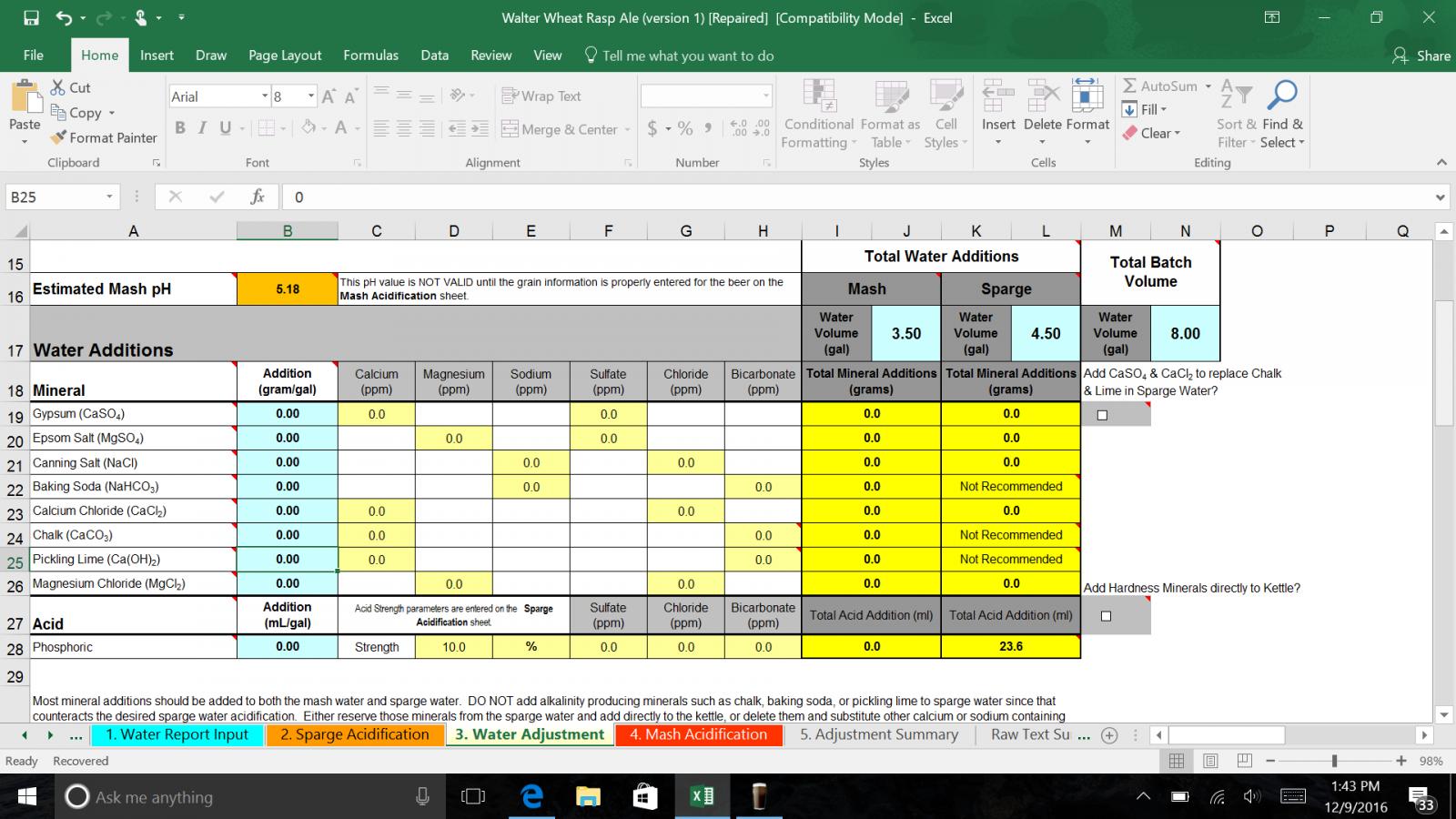
ok peeps, got my water analysis back, brun water downloaded, and I'm confused as I can be. Maybe someone can point me in the right direction. Here is my water profile and grain bill for my upcoming American wheat.
Starting Profile ppm
Ca 46
Mg 4
Na 31
SO4 81
Cl 27
HCO3 105
PH 8.1
Hardness 131
Alkalinity 87
RA 52
SO4/Cl 3.00
Batch Volume 8.00 Gallons
Total Mash 3.50 Gallons
Total Sparge 4.50 Gallons
2 Row Pale Malt 3 lb
white wheat malt 5 lb
Crystal 40L 8.0oz
Melanoiden malt 4.0oz
Acidulated malt 4.0oz
ok peeps, got my water analysis back, brun water downloaded, and I'm confused as I can be. Maybe someone can point me in the right direction. Here is my water profile and grain bill for my upcoming American wheat.
Starting Profile ppm
Ca 46
Mg 4
Na 31
SO4 81
Cl 27
HCO3 105
PH 8.1
Hardness 131
Alkalinity 87
RA 52
SO4/Cl 3.00
Batch Volume 8.00 Gallons
Total Mash 3.50 Gallons
Total Sparge 4.50 Gallons
2 Row Pale Malt 3 lb
white wheat malt 5 lb
Crystal 40L 8.0oz
Melanoiden malt 4.0oz
Acidulated malt 4.0oz
I'm with TripleHopped - your water volumes don't seem to add up correctly. Batch volume should be what's in the fermenter at the end of the brew day. For me, that's 5.5 gallons usually. My mash volumes are usually around 4-4.5 gallons, and my sparge volumes between 5-6 gallons. I fly sparge though, so part of that is mash-out volume (usually 1.5-2 gallons, which leaves about 3 gallons to actually sparge with).
What's your setup like? Batch or fly sparge? Are you in the NOLA area? If so I can possibly help you out with this more in depth, possibly even in person.
I threw the recipe into BeerSmith and it calls for 11.65qt (call it 3 gallons) in the mash (thickness of 1.25qt/lb), and 5.25 gallons sparge. Now this is setup for my system, 5.5 gallon batch with a 1 gallon/hr boil off rate, and I fly sparge. I'm sure you'll want a thinner mash (3.5g sounds good).
So how are you planning to use this Primer? Are you going to dilute down as suggested?

If I wanted to try to "fudge" it with bru'n water, what would be the best way to go about this? It would be easiest if I could do something to the water profile so that I don't have to increase the color of each grain by some arbitrary amount, but even changing my setting from 100% RO to DI only gets me about 0.04 closer to what I'm actually measuring. As a side note, I'm measuring mash pH at room temp with an Omega pH meter that I calibrate while I'm waiting for my sample to cool and which claims an accuracy of +/- 0.01.
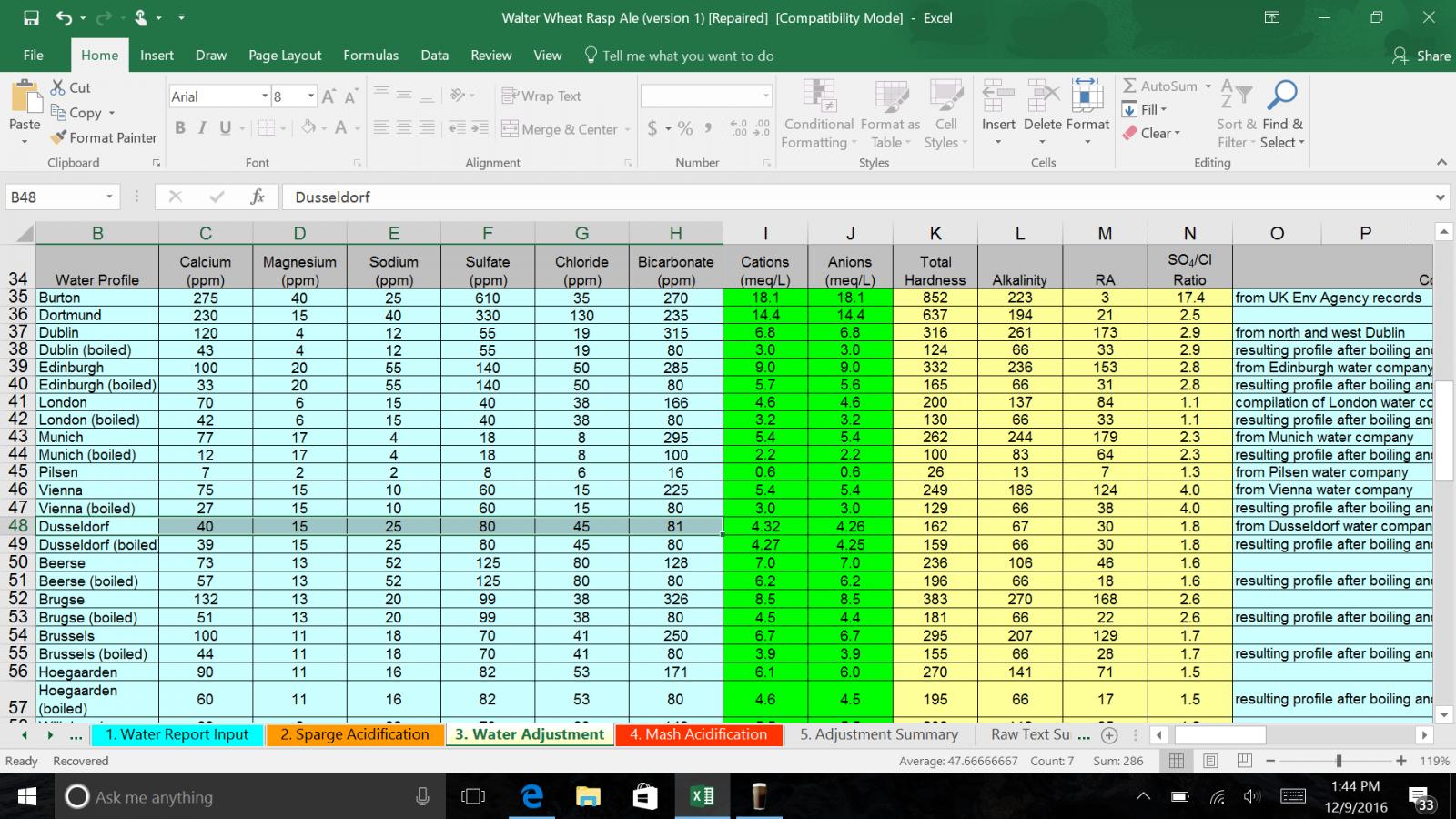
I wouldn't use a Dusseldorf profile for a Hefe. It's a bit more mineralized than I would think would work well. The boiled Munich profile should work better and it's representative of much of Southern Bavaria. That's where a lot of Hefe's are brewed.
I can't believe we are discussing DI pH and buffering in the Primer thread but as we are I'll point out that one must have a value for the grain buffering coefficient (at least the linear one). In another thread (https://www.homebrewtalk.com/showthread.php?p=7841949#post7841949 No. 57) I discuss how incorrect DI pH causes larger errors than incorrect buffering so I'd suggest continuing to measure DI pH whenever you can and just use -40 mEq/kg•pH for a.I continue to wrestle with the calculus needed to arrive at the first coefficient a1*(pH-DIph) . For now I will have to be satisfied with plugging in the DI pH value, without including the grain buffering component in the equation.
Having done what you describe here you simply repeat the process but this time you add a wee bit of acid to the warm water, wait 20 minutes and measure the pH. Since we expect that it will take about 40 mEq of acid to lower the pH of a kg of malt by 1 we'd expect it to take 40/25/10 = 0.16 mEq to lower the pH of 40 grams (1/25th kg) by 1/10 of a point. The challenge here isn't the calculations. It getting 0.16 mEq of acid to add. Obviously the best way to do this is to buy 1 N (1 normal) hydrochloric or sulfuric acid from Hach (or some other supplier) and measure out 0.16 mL. That in itself may be a challenge but an insulin syringe should do the job.The process I use calls for 40g of finely crushed malt, mixed in with 100ml of RO water and then heated to 122F. A sample is later pulled and cooled down to 68F after 20 minutes of mashing. The pH value of the sample is then entered into the software as the actual DI pH value of the grain. Lacking the skills to arrive at a more precise value at the moment I am continuing to learn a great deal about this process with every post that I read. Thank you for sharing your expert knowledge here everyone.
The beer will obviously be more minerally than you might like but other than that will probably be OK.- except screwed up and added the salts to 4 gals and didn't catch it till starting to sparge.
New Milwaukee M102 pH meter calibrated and my first time ever measuring mash pH.
readng: 5.28pH@RT :30 5.22pH@RT end of mash.
If you add 1 cc of 88% lactic acid to 100 mL of DI or RO water you will have a solution that is pretty close to 0.1 N so you would use 1.6 mL of that in order to get 0.16 mEq.
Add that acid to the mash water, wait, and measure pH. Then
a = 4/(pH_shift)
If the malt is typical the pH shift will be about -0.1 and a about -40.
Enter your email address to join: