HossTheGreat
Well-Known Member
- Joined
- Mar 28, 2009
- Messages
- 523
- Reaction score
- 5
I'm still trying to get my head wrapped around all of this water chemistry stuff. I've been reading up on the subject, but a lot of this info is new to me, so I'm turning here for some help.
Currently I'm doing full volume Brew in a Bag mashes. This means I generally mash in rougly 9 gallons of water ~3qt/lb. I've been struggling with very poor light beers (Blondes, Cream Ales, Pale Ales, etc). Generally I pick up, what can best be described as, a raw grain flavor in the finished beer. It very noticible and tends to never drop out over time. From what I read, I believe it could be from tannin extraction during the mash. I have not experienced this off-flavor in any of my darker beers that use roasted or darker crystal malts(ie. Milds, English/American Browns, American Amber)
I've been following the water chemistry primer, using 1g of Calcium Chloride per gallon of water, along with 2% acidulated malt in my grain bill. I however, did not notice any significant change in the overall finished product. Let me also say, that I do not have a pH meter, so I haven't been able to measure of the pH of my mash. However, I'm am currently looking into picking up a pH meter (currently looking at the phep 5) so that I can properly measure it.
I also downloaded the Bru'n spreadsheet and plugged in all of my water info, including all of the adjustments that I made to my water for my last beer using the water primer stickied in this forum. I'm including the screenshots of what Bru'n says. It seems to be somewhat conflicting to what I thought I'd get by following the primer.
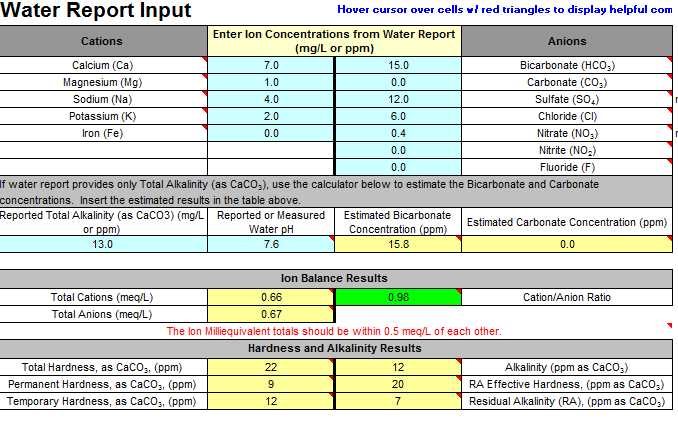
It seems that, while the calcium chloride effectively raises my calcium level to 80ppm, it also raises the chloride level to 133.5. Acording to Bru'n the chloride level should not exceed 100ppm. Is this something that I should be concerned with?
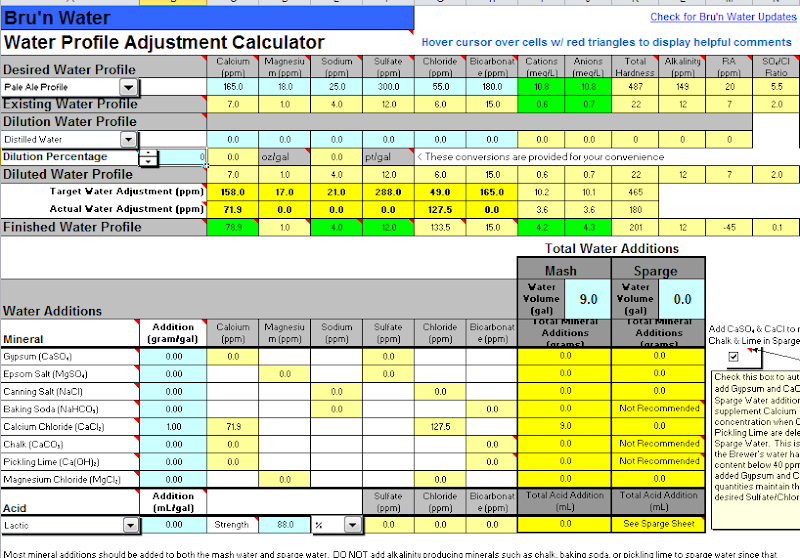
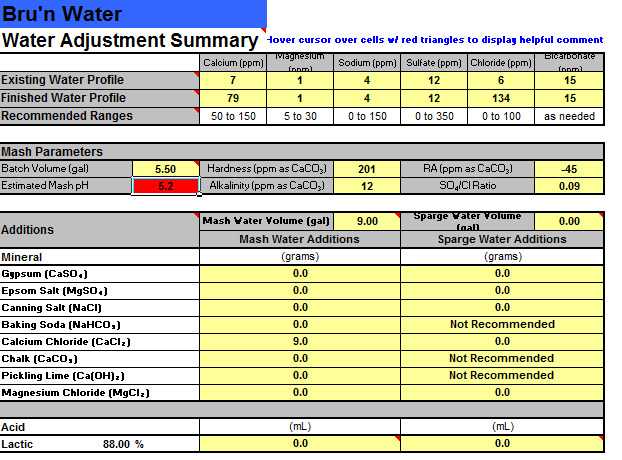
Next, with the 4oz of acidulated malt added to the mash, Bru'n shows the net mash acidity at 79.1 and gives an error that more mash water alkalinity is needed. On the Water Adjustment Summary Page, it shows my Estimated Mash pH at 5.2, but it's highlighted in red.
However, if I remove the acidulated malt altogether, It brings the net mash acidity down to 41.6 (it doesn't give a warning, even though Bru'n says that this number should generally be between 0 and 25) and the Estimated Mash pH on the Water Adjustment Summary Page, while still at 5.2, is then highlighted in green.
I guess I'm just looking for some guidance on what I should do. I'm getting pretty discouraged, turning out one bad light colored beer after another and just need some help. Any advice that you all can offer, I would gladly accept.
Currently I'm doing full volume Brew in a Bag mashes. This means I generally mash in rougly 9 gallons of water ~3qt/lb. I've been struggling with very poor light beers (Blondes, Cream Ales, Pale Ales, etc). Generally I pick up, what can best be described as, a raw grain flavor in the finished beer. It very noticible and tends to never drop out over time. From what I read, I believe it could be from tannin extraction during the mash. I have not experienced this off-flavor in any of my darker beers that use roasted or darker crystal malts(ie. Milds, English/American Browns, American Amber)
I've been following the water chemistry primer, using 1g of Calcium Chloride per gallon of water, along with 2% acidulated malt in my grain bill. I however, did not notice any significant change in the overall finished product. Let me also say, that I do not have a pH meter, so I haven't been able to measure of the pH of my mash. However, I'm am currently looking into picking up a pH meter (currently looking at the phep 5) so that I can properly measure it.
I also downloaded the Bru'n spreadsheet and plugged in all of my water info, including all of the adjustments that I made to my water for my last beer using the water primer stickied in this forum. I'm including the screenshots of what Bru'n says. It seems to be somewhat conflicting to what I thought I'd get by following the primer.

It seems that, while the calcium chloride effectively raises my calcium level to 80ppm, it also raises the chloride level to 133.5. Acording to Bru'n the chloride level should not exceed 100ppm. Is this something that I should be concerned with?

Next, with the 4oz of acidulated malt added to the mash, Bru'n shows the net mash acidity at 79.1 and gives an error that more mash water alkalinity is needed. On the Water Adjustment Summary Page, it shows my Estimated Mash pH at 5.2, but it's highlighted in red.
However, if I remove the acidulated malt altogether, It brings the net mash acidity down to 41.6 (it doesn't give a warning, even though Bru'n says that this number should generally be between 0 and 25) and the Estimated Mash pH on the Water Adjustment Summary Page, while still at 5.2, is then highlighted in green.

I guess I'm just looking for some guidance on what I should do. I'm getting pretty discouraged, turning out one bad light colored beer after another and just need some help. Any advice that you all can offer, I would gladly accept.


