Yes.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Pics of Yeast under my new microscope
- Thread starter passedpawn
- Start date

Help Support Homebrew Talk - Beer, Wine, Mead, & Cider Brewing Discussion Forum:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
I got my microscope a couple days ago and have been geeking out and having a ball doing it. Very cool to see my yeasties up close.
Can anyone suggest a good reference online or book for identifying different types of yeast cultures from each other and from bacteria? I have the Yeast book from Jamil and White but would like something a little more in depth. Thanks for any suggestions!
I took these pics of wild Reinart ale and a wild Avery beer with my phone camera lens held to the eye piece. It's a work in progress
Can anyone suggest a good reference online or book for identifying different types of yeast cultures from each other and from bacteria? I have the Yeast book from Jamil and White but would like something a little more in depth. Thanks for any suggestions!
I took these pics of wild Reinart ale and a wild Avery beer with my phone camera lens held to the eye piece. It's a work in progress


PoppinCaps
Well-Known Member
Bacteria is easy, you can really only see them at 400x, they're super tiny compared to the yeast and dancing around. Hard to distinguish from small particles with Brownian motion, but it's obvious when there's an infection. Identifying different yeast is a tough one if you're only talking Saccharomyces, as they're roughly identical in looks, colony formation and color, etc. If you're talking wild yeast captured, some yeast will have hyphae (like mushroom "roots"), spores, crazy colors, etc. Then you could use an agar plate to distinguish soft white colonies from the crazy ones. Sorry, I don't have a good suggestion for reading material, but any fungal text is a good start.
Nice work with the camera phone!
Nice work with the camera phone!
Damn robh you took those pics with your phone? That's incredible.
I have the same desire as you, identifying yeast and bacteria. There's some good blogs out there with info. I'm on the road now (San Francisco) I'll post links when I get back.
I have the same desire as you, identifying yeast and bacteria. There's some good blogs out there with info. I'm on the road now (San Francisco) I'll post links when I get back.
Thanks for the info PoppinCaps. I'll check and see if my local library has any good fungal texts.
Passedpawn - I was pleasantly surprised at the quality too! I definitely have room for improvement, but I'm happy with that clarity considering how easy it was to do. I have an Android Evo with an 8 megapixel camera. The autofocus helps a lot. Please do send any links or references that you think might be helpful. The info you and others have provided on this forum have been a great help already! Thanks.
Passedpawn - I was pleasantly surprised at the quality too! I definitely have room for improvement, but I'm happy with that clarity considering how easy it was to do. I have an Android Evo with an 8 megapixel camera. The autofocus helps a lot. Please do send any links or references that you think might be helpful. The info you and others have provided on this forum have been a great help already! Thanks.
Here's a couple new pics I snapped today at school. They looked much better on the comp that snaps the photos but I guess the data transfer or the program I'm using to modify and crop do something to the quality. Also I'd like to note I don't know how accurate the scale is, but it can be used for size reference. I took all these pictures at the same magnification.
One pic is a wild yeast colony I pulled off a Lins Cupric Sulfate Medium. I think there where 3 total decent sized colonies. One separate and two colonies that merged together to equal about the size of the single. I have images of WLP510 that I am in the process of growing up from frozen stock as of yesterday.
Here's the yeast that grew on the LCSM.

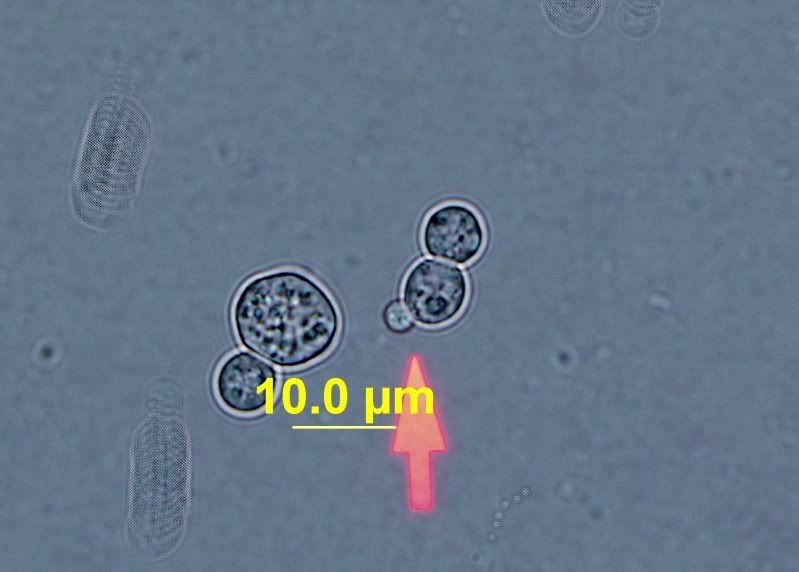
Here's WLP510 for reference. You can see a nice tiny bud in the second picture.

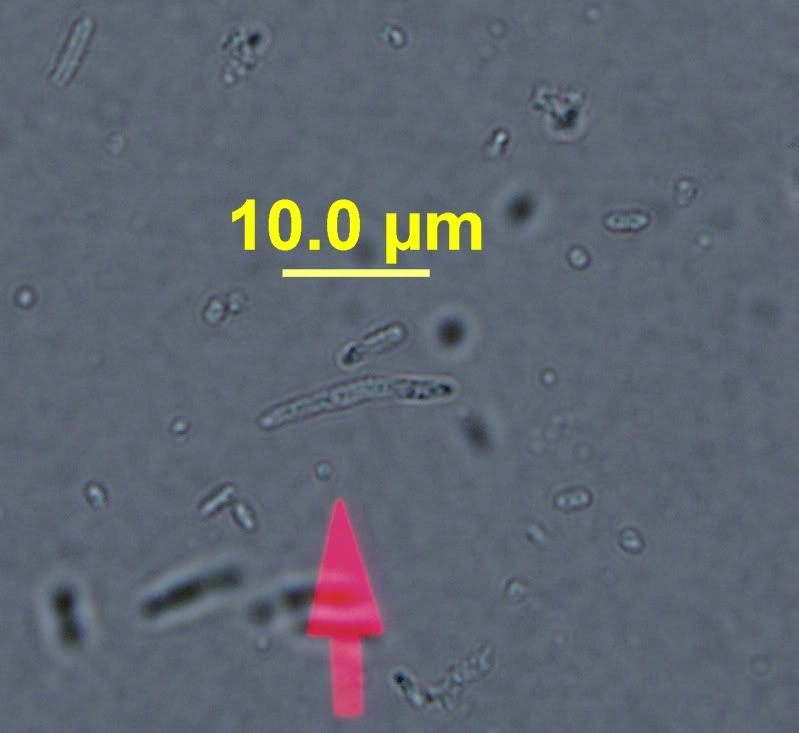
Then here's some of what I suspect to be brett. It was isolated and grown from a unknown culture on WLD medium.


One pic is a wild yeast colony I pulled off a Lins Cupric Sulfate Medium. I think there where 3 total decent sized colonies. One separate and two colonies that merged together to equal about the size of the single. I have images of WLP510 that I am in the process of growing up from frozen stock as of yesterday.
Here's the yeast that grew on the LCSM.

Here's WLP510 for reference. You can see a nice tiny bud in the second picture.


Then here's some of what I suspect to be brett. It was isolated and grown from a unknown culture on WLD medium.



dstar26t
If it's worth doing, it's worth overdoing
Those pics are awesome. I'm finding that a microscope is a very important part of my brewery, not just another toy to play with.
I'm brewing a big Stout on Saturday and made a starter for it this past Tuesday. Looking for 765 Billion cells so I put 3 vials (WLP001) in a 4L starter. It was finished this morning so I did a cell count. Only 394 Billion cells! My microscope has officially saved it's first beer. I can easily supplement with some dry yeast now to get the correct pitching rate. Before the microscope, the only tool available to me was Jamil's calculator which it seems doesn't by itself insure the correct cell count.
I'm brewing a big Stout on Saturday and made a starter for it this past Tuesday. Looking for 765 Billion cells so I put 3 vials (WLP001) in a 4L starter. It was finished this morning so I did a cell count. Only 394 Billion cells! My microscope has officially saved it's first beer. I can easily supplement with some dry yeast now to get the correct pitching rate. Before the microscope, the only tool available to me was Jamil's calculator which it seems doesn't by itself insure the correct cell count.
Those pics are awesome. I'm finding that a microscope is a very important part of my brewery, not just another toy to play with.
I'm brewing a big Stout on Saturday and made a starter for it this past Tuesday. Looking for 765 Billion cells so I put 3 vials (WLP001) in a 4L starter. It was finished this morning so I did a cell count. Only 394 Billion cells! My microscope has officially saved it's first beer. I can easily supplement with some dry yeast now to get the correct pitching rate. Before the microscope, the only tool available to me was Jamil's calculator which it seems doesn't by itself insure the correct cell count.
How old were the vials? There's around 100B cells in a vial, so either your's weren't very viable or you had very little growth. How much DME did you add to the 4L?
I use mine on every starter. 1 drop of starter and a couple of minutes of counting.
I've played some more with video, but I haven't captured budding yeast yet. Once I do that, I'll time-lapse it and post here.
I've played some more with video, but I haven't captured budding yeast yet. Once I do that, I'll time-lapse it and post here.
dstar26t
If it's worth doing, it's worth overdoing
Vials dated 7/11/12 and kept cold at homebrew shop. I drove the vials home from the shop in a cooler with ice. Let them warm to room temp before pitching. 3810 grams water and 381 grams DME on stir plate for 36 hours at 66ºF. Treated the water with K2S2O5 at the recommended rate (.44g/20gal) and used the recommended amount of Wyeast Yeast Nutrient (2.2g/5gal).
I'm at a loss as to why they didn't multiply more, glad I checked though!
I'm at a loss as to why they didn't multiply more, glad I checked though!
dstar26t said:Vials dated 7/11/12 and kept cold at homebrew shop. I drove the vials home from the homebrew shop in a cooler with ice. Let them warm to room temp before pitching. 3810 grams water and 381 grams DME on stir plate for 36 hours at 66ºF. Treated the water with K2S2O5 at the recommended rate (.44g/20gal) and used the recommended amount of Wyeast Yeast Nutrient (2.2g/5gal).
I'm at a loss as to why they didn't multiply more, glad I checked though!
You might want to double check your math and your count. Seems too low based on the inputs, but I'm very curious to see info comparing actual counts to estimated growth values.
dstar26t
If it's worth doing, it's worth overdoing
I counted 2 separate samples. One was 16:1 dilution and counted 5 of the 25 grids. The number of counted cells was low since I expected more and diluted for what I expected. 123 yeast cells total counted in 5 grids x 5 x 16 x 10000 x 4000 = 394 Billion cells
dstar26t
If it's worth doing, it's worth overdoing
I have some Alkaline Methylene Violet but didn't bother using it since I figured viability would be pretty high since it's a starter that's 36 hours old. I didn't bother checking the yeast in the vials either since they were a few days old. Something fishy happened.
I have some Alkaline Methylene Violet
Did you get that from White labs or did you buy the powder and make it yourself?
Have you guys counted cells right out of the pack? I'm gonna look at that tonight. I put a drop on the scope last night and it needs a LOT of dilution... didn't have time to play then, but tonight I will.
dstar26t
If it's worth doing, it's worth overdoing
Did you get that from White labs or did you buy the powder and make it yourself?
I got it straight from White Labs because I do get lazy from time to time.
dstar26t
If it's worth doing, it's worth overdoing
Have you guys counted cells right out of the pack? I'm gonna look at that tonight. I put a drop on the scope last night and it needs a LOT of dilution... didn't have time to play then, but tonight I will.
For a White Labs vial, You'd need to dilute it like 150:1 and that's if you can mix the vial up thoroughly. A Wyeast pack would be about 1/3 the dilution.
bruin_ale
Well-Known Member
dstar, can I ask why you treated the starter water with potassium metabisulfite? I know that will neutralize chlorine and thus polyphenols in the finished product but I never use it on my starters because it's such a small amount and I'm fermenting warm and producing all kinds of off-flavors with the starter that I really don't care about one more. Wouldn't the kmeta inhibit yeast growth or did you wait a certain amount of time for it to neutralize before you pitched?
dstar26t
If it's worth doing, it's worth overdoing
That's an interesting point and something I've thought about. It gets added to the water before it's boiled for 15 minutes and cooled. I wonder if it may have an effect on the yeast? Ever since I had what I thought was a chlorine/chloramine related flavor in a batch a couple years ago, I treat all water with K2S2O5.dstar, can I ask why you treated the starter water with potassium metabisulfite? I know that will neutralize chlorine and thus polyphenols in the finished product but I never use it on my starters because it's such a small amount and I'm fermenting warm and producing all kinds of off-flavors with the starter that I really don't care about one more. Wouldn't the kmeta inhibit yeast growth or did you wait a certain amount of time for it to neutralize before you pitched?
That's an interesting point and something I've thought about. It gets added to the water before it's boiled for 15 minutes and cooled. I wonder if it may have an effect on the yeast? Ever since I had what I thought was a chlorine/chloramine related flavor in a batch a couple years ago, I treat all water with K2S2O5.
Do you have time to decant your starter and repitch into wort without the K2S2O5? Interested in hearing if you get the growth you're expecting.
Curtis2010
Well-Known Member
- Joined
- Dec 6, 2011
- Messages
- 1,875
- Reaction score
- 640
Oh boy, more geeky stuff to buy for brewing...something to look forward to for next brew season!
Oh boy, more geeky stuff to buy for brewing...something to look forward to for next brew season!
There are brewing seasons? For me, brewing season starts every Saturday and ends Sunday.
dstar26t
If it's worth doing, it's worth overdoing
Do you have time to decant your starter and repitch into wort without the K2S2O5? Interested in hearing if you get the growth you're expecting.
No but this result definitely calls for a controlled experiment.
What kind of scope? any tips on the basics for me buying a used one on ebay?
dstar26t
If it's worth doing, it's worth overdoing
bruin_ale
Well-Known Member
^ would love to see that stained to make out what all the creatures are. I see some different shapes in there, but not educated enough to know what I'm looking at.
Passedpawn,
To answer the question you asked on like page 7 or 8 about phase contrast. The scope I am using at school is capable, but I do not have the necessary filters to do so. At least I don't think I do. I looked through the manual and online. It's a separate add on kit I do believe. I can do florescence though. I might have mentioned that, I just don't have the specific dyes needed to do the imaging.
To answer the question you asked on like page 7 or 8 about phase contrast. The scope I am using at school is capable, but I do not have the necessary filters to do so. At least I don't think I do. I looked through the manual and online. It's a separate add on kit I do believe. I can do florescence though. I might have mentioned that, I just don't have the specific dyes needed to do the imaging.
BradleyBrew
Well-Known Member
this is one thing I have not researched about homebrewing. What are the benefits of this scope? Cell counts? Looking for bacteria? I need enlightened lol
cheezydemon3
Well-Known Member
Cell counts.
Very cool PP, but don't be offended, I never will feel the need for one.
Very cool PP, but don't be offended, I never will feel the need for one.
Well, I've been holding my breath waiting for my local fish shop to replenish their methylene blue, but I guess they're never gonna. So I bit the bullet and ordered the violet (and blue) stains from White Labs. Yep, stepping up my game baby!
I just killed a fresh batch of Wyeast 1762, not sure how, but I've got to start doing viability. Woot!
I just killed a fresh batch of Wyeast 1762, not sure how, but I've got to start doing viability. Woot!
Cell counts.
Very cool PP, but don't be offended, I never will feel the need for one.
I understand. I've gotten the EXACT same comments regarding homebrewing!
I'm a nerd through-and-through. Sad, really, but I'm just as god made me.
Most beneficial for cell counts. Its tough to see bacteria especially when mixed up with yeast. You'd have to get the bacteria separately really and dye it to properly see its morphology.
More pics.
Here's a sample of yeast straight from a pouch of Wyeast Belgain Abby II #1762. Chock full of nuts! First video is of the undiluted yeast. I change the scope optics from 40x -> 100x -> 200x -> 400x. I have to play a bit with focus and illumination, especially when I go to 100x.
And here's the same yeast diluted 50:1. I cleaned my camera lens and adjusted the video colors and played around with the scope lighting between videos, so they look very different. Sorry about that - still getting the hang of it. Note the little things squiggling around there. I don't know if those are bacteria or just some tiny material affected by brownian motion.
Based on the dilution and the number of cells there, I get 82.5 billion in the pouch. I only counted one cell area, so lots of possible error there. My dilution is using disposable pipettes, could be quite a bit of error there too. But the result is very close to what Wyeast states. BTW, I've measured about 150ml from a smack pack after the internal nutrient pouch is broken and mixed in. Here's the spreadsheet I made to do my counts... note the Total Cells in Flask (which in this case is the 150ml pouch).

Here's a sample of yeast straight from a pouch of Wyeast Belgain Abby II #1762. Chock full of nuts! First video is of the undiluted yeast. I change the scope optics from 40x -> 100x -> 200x -> 400x. I have to play a bit with focus and illumination, especially when I go to 100x.
And here's the same yeast diluted 50:1. I cleaned my camera lens and adjusted the video colors and played around with the scope lighting between videos, so they look very different. Sorry about that - still getting the hang of it. Note the little things squiggling around there. I don't know if those are bacteria or just some tiny material affected by brownian motion.
Based on the dilution and the number of cells there, I get 82.5 billion in the pouch. I only counted one cell area, so lots of possible error there. My dilution is using disposable pipettes, could be quite a bit of error there too. But the result is very close to what Wyeast states. BTW, I've measured about 150ml from a smack pack after the internal nutrient pouch is broken and mixed in. Here's the spreadsheet I made to do my counts... note the Total Cells in Flask (which in this case is the 150ml pouch).

Last edited by a moderator:
The way I count is the short count method. You count the four corners and the center block for the 5th then multiply by 5 for an approximation of the whole grid. Then you do your normal multiplication by the dilution factor and the 10,000 for the volume to make it equal to 1ml.
bierhaus15
Well-Known Member
- Joined
- Aug 4, 2008
- Messages
- 1,949
- Reaction score
- 446
Can someone give me a short rundown on the cost and type of equipment I would need to get into this? I have a very old scope that I haven't touched since HS, but I doubt it would work for this. I know my local college sells used lab equipment a few times a year.
wegz15
Well-Known Member
I had a good scope laying aground. I bought a used hemocytometer off ebay for about 20
Can someone give me a short rundown on the cost and type of equipment I would need to get into this? I have a very old scope that I haven't touched since HS, but I doubt it would work for this. I know my local college sells used lab equipment a few times a year.
If you look through this thread I think it's been discussed. Let me know what exact questions you have and I'll try to help.
wegz15
Well-Known Member
So... working on a two hearted clone. Took some dregs from a 3 two hearted bottles. They're a span of a couple weeks drinking... covered with the cap. 60% viability. 1.6 million cells/ml. 1.6*.6=960000 cells/ml.
Stir plate is stirring away. Lets see how this goes.
Stir plate is stirring away. Lets see how this goes.
Similar threads
- Replies
- 2
- Views
- 645


