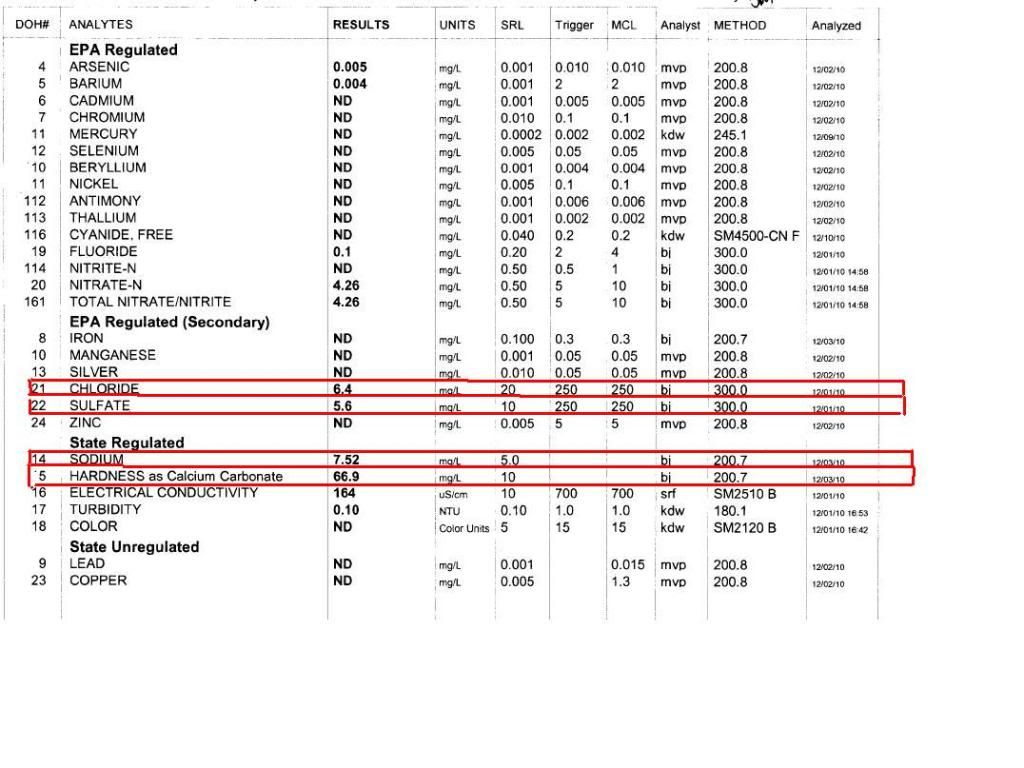
It really is terrible of these water authorities. Don't they understand that we care much more about our beer than we do about their stupid heavy metal poisoning? Anyway, yes, you are missing info about calcium, magnesium and alkalinity - the three most important parts of a water report for a brewer. The units "as calcium chloride" refer to the number of charges on an ion expressed as if they came from the calcium in calcium carbonate which was dissolved by carbonic acid. If 100 mg is put into a liter of water and CO2 bubbled through until the chalk is all dissolved and the water clear then that water would contain 2 mEq/L Calcium ions and 2 mEq/K bicarbonate ions (approximately). The molecular weight of calcium carbonate is 100 so 1 mg/L is 1 millimole/L containing 1 mmol/L calcium and 1 mmol/L carbonate. The carbonate reacts with CO2 and water thus: CO3-- + CO2 + H2O ---> 2HCO3- so that the 1 mmol/L CO3-- becomes 2 mmol/L HCO3-. The equivalence is the number of charges i.e. 1 mmol/L CO3-- is 2 meq/L of that and 1 mmol/L of Ca++ is 2 meq/l Ca++. And the 2 mmol/L bicarbonate is 2 meq/L. As the idea was to have alkalinity and hardness numbers representative of the amount of CaCO3 dissolved by CO2 (which is the mechanism used in nature) the "as CaCO3" unit, defined as being 50 times the equivalence was adopted. Thus if 100 mg chalk is dissolved in 1 L of water using CO2 the hardness will be 100 ppm as CaCO3 and the alkalinity will be approximately 100 ppm as CaCO3.
When they tell you the hardness is 66.9 ppm as CaCO3 they are saying that the charges on the calcium plus those on the magnesium (by definition these are the only components of hardness) total 66.9/50 meq/L but they are not telling you how much of this is from calcium and how much from magnesium.
Yes, it is possible to estimate the alkalinity by the fact that the electric charge contributed by it and the other anions must be balanced by the electric charge on the hardness ions (and that's what hardness in "as CaCO3" units divided by 50 represents) plus the charge on the other cations. For this report the alkalinity will be approximately 54 ppm as CaCO3. There is no way, however, to tell how much magnesium there is relative to the amount of calcium other than to look at bertmurphy's post and assume that the representation there that magnesium content is relatively small is correct.