uberg33k
Well-Known Member
Hey all!
I was just playing Bru'n Water trying to get a recipe to match up as closely as possible to the original. I realized after playing with the sheets for a while, I might have gone full tweaker and overdone it, but the numbers seem to match up. I was just hoping for a sanity check.
The recipe: http://www.brewtoad.com/recipes/farmhouse-rye-ale
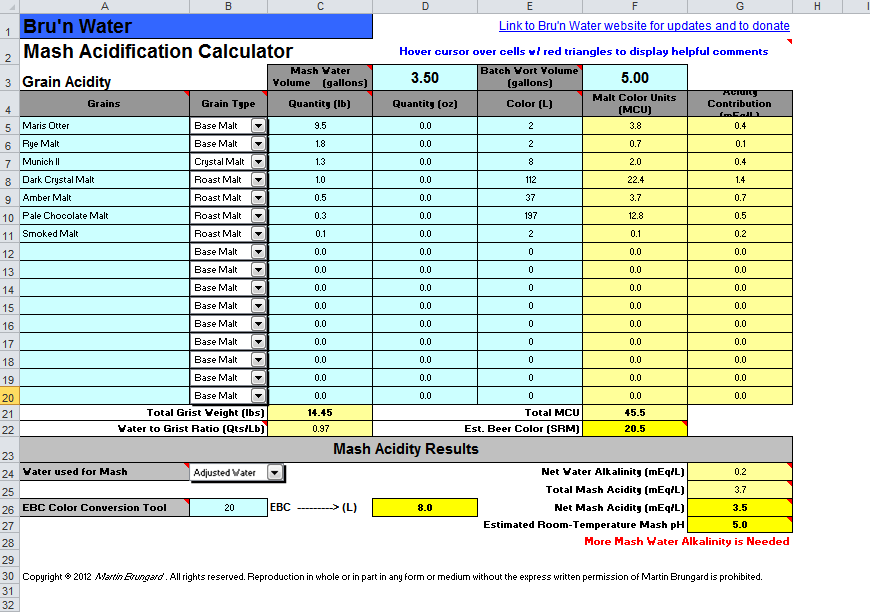
I do full volume BIAB (mostly due to laziness), so I'm starting at 7.75gal H2O.
My adjustments come out to:
So that means adding...
Epsom Salt: 3.5g
Baking Soda: 1.5g
Calcium Chloride: 1.5g
Pickling Lime: 3.5g
Magnesium Chloride: 0.5g
Does that look right? That just seems to me to be a lot of additions to get the mash pH in line and approximate the water profile in Denmark. I just want to make sure I'm not making a mess before proceeding. Any suggestions?
Thanks!
I was just playing Bru'n Water trying to get a recipe to match up as closely as possible to the original. I realized after playing with the sheets for a while, I might have gone full tweaker and overdone it, but the numbers seem to match up. I was just hoping for a sanity check.
The recipe: http://www.brewtoad.com/recipes/farmhouse-rye-ale
I do full volume BIAB (mostly due to laziness), so I'm starting at 7.75gal H2O.
My adjustments come out to:
Code:
Ca Mg Na SO4 Cl HCO3
Existing Water Profile 9 1 6 9 7 19
Finished Water Profile 87 15 20 55 38 251
Mash Parameters
Batch Volume (gal) 5.00 Hardness (ppm as CaCO3) 279 RA (ppm as CaCO3) 137
Estimated Mash pH 5.2 Alkalinity (ppm as CaCO3) 208 SO4/Cl Ratio 1.47So that means adding...
Epsom Salt: 3.5g
Baking Soda: 1.5g
Calcium Chloride: 1.5g
Pickling Lime: 3.5g
Magnesium Chloride: 0.5g
Does that look right? That just seems to me to be a lot of additions to get the mash pH in line and approximate the water profile in Denmark. I just want to make sure I'm not making a mess before proceeding. Any suggestions?
Thanks!