runningweird
Well-Known Member
Ian Weir
ENVI 220 Project Report
Tom Marsik
4/12/12
Calculations:
How many BTU in 1 kWh
It Takes 1 BTU/hour to increase 1lb of water 1 degree F
1 gallon of water weighs 8.3lbs
So it takes 8.3BTU/hr to heat 1 gallon of water by 1 degree
To convert BTUs to kWh we find the following:
1 BTU = 1.055 kilojoules
1 joule/second = 1 watt
Or
1 joule = 1 watt/second
There are 3600 seconds per hour
1kWh = 1000x3600watt.seconds = 1000x3600 joules = 3600kilojoules = 3600kilojoues/1.055kilojoules/BTU = 3412.3BTU
1kWh = 3412.4 BTU/hr
So to heat one gallon of water we use the equation (quantity of water) x (temperature raise needed)x8.3BTU/hr = Energy needed
Theoretical Calculations:
Propane Burner:
Burner calculations basics: operating under the assumption that the burner is 100% efficient at
Transferring energy and there is 0 heat loss from the vessel
220,000BTU/hr
60 minutes/hour
So (220,000BTU/hr)/60minutes/hr)=3666BTU/minute
So to heat one gallon of water
we need to find BTU per minute and how long to heat 1 gallon of water from 60 to 212
so using the formula (amount of water)x(Degree increase)x8.3BTU = Energy Needed
1gallon x 152degrees/gallon x 8.3BTU/degree = 1261.6 BTU
The burner can produce 3666 BTU/minute so
1261.6BTU/3666BTU/Minute = .344 minutes to heat 1 gallon of water to boiling from 60 degrees F
COST
To fill a 20 pound propane tank locally costs $17 so the propane costs ₵85 per pound of propane
1 pound of propane is capable of producing 21,622BTU taken from http://www.propanegas.ca/FileArea/PGAC/Propane properties.pdf
So to heat one gallon of water requires 1261.6BTU and there are 21622BTU in one pound of propane
1261.6BTU/21,622 BTU/lb = .058lb of propane to heat one gallon of water
.58lb x ₵85/lb=₵4.95 to heat one gallon of water to boiling
And would take .344 minutes to do so
Electrical Element:
Electrical
Again, we are assuming in this set of calculations that they are 100% efficient in heat transfer and there is 0 energy loss
The boil kettle and Heat exchanger are both equipped with a single 240v 5.5kW heating element
1kW=3412.3BTU so
5.5kW x 3412.3BTU/kW = 18,767BTU
In one hour the heating elements are able to each provide a maximum of 18,767 BTU/hr to the liquid.
To bring 1 gallon of water from 60 degrees to 212 we use the equation
1gallon x 152degrees/gallon x 8.3BTU/degree = 1261.6 BTU
And to find the kWh used:
1kWh = 3412.3BTU
So 1261.6BTU/3412.3BTU/kWh = .369kWh
To calculate cost:
1kWh costs ₵6.99
₵6.99/kWh x .369kWh=₵2.58 to heat one gallon of water to boiling in a perfect system
(1261.6BTU/ 18,767BTU/hr)x60minutes/hr = 4.03 minutes
So it would take 4.03 minutes to boil 1 gallon of water using the electrical system
Real World Experiments
Propane experiment:
To heat 6 gallons of water from 70 degrees to boiling: (6)x(142)x8.3BTU=7071.6
1. Propane Burner turned on maximum
2. Kettle Full of water placed on burner stand
3. Expected Time to Boil according to theoretical calculations: 2.064 minutes
4. Actual Time to boil: 25.5 minutes
5. Actual burner efficiency is 2.064/25.5 = .08 or 8%
Electric Experiment:
To Heat 6 Gallons of water from 86 degrees to boiling: (6)x(126)x(8.3BTU)=6274.8
1. Kettle Filled to 6 gallons
2. Element turned on full
3. Expected time to boil according to theoretical calculations:20.06 minutes
4. Time to boil:26.44 minutes
5. Actual element efficiency is 20.06minutes/26.44minutes = .758 or 75.8% efficient
Cost Per BTU
Propane
20 lb PROPANE tank refill costs $17
21,622 BTU per pound so total BTU in one 20lb Tank= 21,622BTU/lb x 20lb = 432,440BTU
$17/432,440BTU= $.0000393/BTU
Electricity
1kWh of electricity costs ₵6.99 or $.0699
1kWh = 3412.3BTU
$.0699/3412.3BTU = $.00002/BTU
Actual Cost Calculations Per 5 gallon Batch
For this batch of beer I used 10 pounds of grain for which I heated 1.25 quarts of water per pound.
10lbs x 1.25qt/lb = 12.5 quarts /4quarts/gallon = 3.125 gallons of mash water
Propane:
Mash Calulations: heating water from 60 degrees F to 163 degrees F
(3.125)x(103)x(8.3BTU)=2761.56BTU
Compensating for 8% efficiency = 2761.56BTU/.08= 33394.5BTU
Sparge Calculation: Heat water from 60 degrees F to 173 degrees F
(4.875)x(112)x(8.3BTU)= 4531.8BTU
Compensating for 8% efficiency =4531.8BTU/.08=46647.5BTU
Boil: Heating from 168 degrees F to 212 degrees F
(6.75)x(44)x(8.3BTU) = 2465.1BTU
Compensating for 8% efficiency =2465.1BTU/.08 = 30,813BTU
Loss of 55 degrees per hour = (6.75)x(55)x(8.3BTU) = 3081.37BTU
Compensating for 8% efficiency=3081.37BTU/.08 = 38517BTU
Total BTU for one Brew Session: 149372BTU
Total cost = 149372BTU x $.0000393/BTU = $5.87 per 5 gallon batch of beer
Electrical:
Mash Calulations: heating water from 60 degrees F to 163 degrees F
(3.125)x(103)x(8.3BTU)=2761.56BTU
Compensating for 75.8% efficiency = 2761.56BTU/.758 = 3524.5BTU
Sparge Calculation: Heat water from 60 degrees F to 173 degrees F
(4.875)x(112)x(8.3BTU)= 4531.8BTU
Compensating for 75.8% efficiency =4531.8BTU/.758 = 5978.6BTU
Boil: Heating from 168 degrees F to 212 degrees F
(6.75)x(44)x(8.3BTU) = 2465.1BTU
Compensating for 75.8% efficiency =2465.1BTU/.758=7887.4BTU
Loss of 55 degrees per hour = (6.75)x(55)x(8.3BTU) = 3081.37BTU
Compensating for 75.8% efficiency=3081.37BTU/.758=4065.13BTU
Total = 21455.63BTU
Cost per 5 gallon batch = 21455.63BTUx$.00002/BTU = $.429/batch
I am sure you guys will rip my work apart, which is what I need - also, how do I calculate the amount of energy used during the boil?
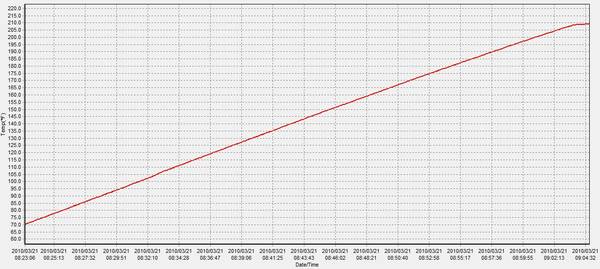
i tried to do it by finding out how much heat was lost over an hour from a kettle heated to boiling then left for an hour - which I found was 55 degrees so I calculated the additional energy needed to heat the kettle that much more during the boil.
i am a terrible procrastinator -this project is due tomorrow so any corrections would be greatly appreciated.
I have the 5 page project proposal done and typed up - if I need to change a few values I can easily do so.
ENVI 220 Project Report
Tom Marsik
4/12/12
Calculations:
How many BTU in 1 kWh
It Takes 1 BTU/hour to increase 1lb of water 1 degree F
1 gallon of water weighs 8.3lbs
So it takes 8.3BTU/hr to heat 1 gallon of water by 1 degree
To convert BTUs to kWh we find the following:
1 BTU = 1.055 kilojoules
1 joule/second = 1 watt
Or
1 joule = 1 watt/second
There are 3600 seconds per hour
1kWh = 1000x3600watt.seconds = 1000x3600 joules = 3600kilojoules = 3600kilojoues/1.055kilojoules/BTU = 3412.3BTU
1kWh = 3412.4 BTU/hr
So to heat one gallon of water we use the equation (quantity of water) x (temperature raise needed)x8.3BTU/hr = Energy needed
Theoretical Calculations:
Propane Burner:
Burner calculations basics: operating under the assumption that the burner is 100% efficient at
Transferring energy and there is 0 heat loss from the vessel
220,000BTU/hr
60 minutes/hour
So (220,000BTU/hr)/60minutes/hr)=3666BTU/minute
So to heat one gallon of water
we need to find BTU per minute and how long to heat 1 gallon of water from 60 to 212
so using the formula (amount of water)x(Degree increase)x8.3BTU = Energy Needed
1gallon x 152degrees/gallon x 8.3BTU/degree = 1261.6 BTU
The burner can produce 3666 BTU/minute so
1261.6BTU/3666BTU/Minute = .344 minutes to heat 1 gallon of water to boiling from 60 degrees F
COST
To fill a 20 pound propane tank locally costs $17 so the propane costs ₵85 per pound of propane
1 pound of propane is capable of producing 21,622BTU taken from http://www.propanegas.ca/FileArea/PGAC/Propane properties.pdf
So to heat one gallon of water requires 1261.6BTU and there are 21622BTU in one pound of propane
1261.6BTU/21,622 BTU/lb = .058lb of propane to heat one gallon of water
.58lb x ₵85/lb=₵4.95 to heat one gallon of water to boiling
And would take .344 minutes to do so
Electrical Element:
Electrical
Again, we are assuming in this set of calculations that they are 100% efficient in heat transfer and there is 0 energy loss
The boil kettle and Heat exchanger are both equipped with a single 240v 5.5kW heating element
1kW=3412.3BTU so
5.5kW x 3412.3BTU/kW = 18,767BTU
In one hour the heating elements are able to each provide a maximum of 18,767 BTU/hr to the liquid.
To bring 1 gallon of water from 60 degrees to 212 we use the equation
1gallon x 152degrees/gallon x 8.3BTU/degree = 1261.6 BTU
And to find the kWh used:
1kWh = 3412.3BTU
So 1261.6BTU/3412.3BTU/kWh = .369kWh
To calculate cost:
1kWh costs ₵6.99
₵6.99/kWh x .369kWh=₵2.58 to heat one gallon of water to boiling in a perfect system
(1261.6BTU/ 18,767BTU/hr)x60minutes/hr = 4.03 minutes
So it would take 4.03 minutes to boil 1 gallon of water using the electrical system
Real World Experiments
Propane experiment:
To heat 6 gallons of water from 70 degrees to boiling: (6)x(142)x8.3BTU=7071.6
1. Propane Burner turned on maximum
2. Kettle Full of water placed on burner stand
3. Expected Time to Boil according to theoretical calculations: 2.064 minutes
4. Actual Time to boil: 25.5 minutes
5. Actual burner efficiency is 2.064/25.5 = .08 or 8%
Electric Experiment:
To Heat 6 Gallons of water from 86 degrees to boiling: (6)x(126)x(8.3BTU)=6274.8
1. Kettle Filled to 6 gallons
2. Element turned on full
3. Expected time to boil according to theoretical calculations:20.06 minutes
4. Time to boil:26.44 minutes
5. Actual element efficiency is 20.06minutes/26.44minutes = .758 or 75.8% efficient
Cost Per BTU
Propane
20 lb PROPANE tank refill costs $17
21,622 BTU per pound so total BTU in one 20lb Tank= 21,622BTU/lb x 20lb = 432,440BTU
$17/432,440BTU= $.0000393/BTU
Electricity
1kWh of electricity costs ₵6.99 or $.0699
1kWh = 3412.3BTU
$.0699/3412.3BTU = $.00002/BTU
Actual Cost Calculations Per 5 gallon Batch
For this batch of beer I used 10 pounds of grain for which I heated 1.25 quarts of water per pound.
10lbs x 1.25qt/lb = 12.5 quarts /4quarts/gallon = 3.125 gallons of mash water
Propane:
Mash Calulations: heating water from 60 degrees F to 163 degrees F
(3.125)x(103)x(8.3BTU)=2761.56BTU
Compensating for 8% efficiency = 2761.56BTU/.08= 33394.5BTU
Sparge Calculation: Heat water from 60 degrees F to 173 degrees F
(4.875)x(112)x(8.3BTU)= 4531.8BTU
Compensating for 8% efficiency =4531.8BTU/.08=46647.5BTU
Boil: Heating from 168 degrees F to 212 degrees F
(6.75)x(44)x(8.3BTU) = 2465.1BTU
Compensating for 8% efficiency =2465.1BTU/.08 = 30,813BTU
Loss of 55 degrees per hour = (6.75)x(55)x(8.3BTU) = 3081.37BTU
Compensating for 8% efficiency=3081.37BTU/.08 = 38517BTU
Total BTU for one Brew Session: 149372BTU
Total cost = 149372BTU x $.0000393/BTU = $5.87 per 5 gallon batch of beer
Electrical:
Mash Calulations: heating water from 60 degrees F to 163 degrees F
(3.125)x(103)x(8.3BTU)=2761.56BTU
Compensating for 75.8% efficiency = 2761.56BTU/.758 = 3524.5BTU
Sparge Calculation: Heat water from 60 degrees F to 173 degrees F
(4.875)x(112)x(8.3BTU)= 4531.8BTU
Compensating for 75.8% efficiency =4531.8BTU/.758 = 5978.6BTU
Boil: Heating from 168 degrees F to 212 degrees F
(6.75)x(44)x(8.3BTU) = 2465.1BTU
Compensating for 75.8% efficiency =2465.1BTU/.758=7887.4BTU
Loss of 55 degrees per hour = (6.75)x(55)x(8.3BTU) = 3081.37BTU
Compensating for 75.8% efficiency=3081.37BTU/.758=4065.13BTU
Total = 21455.63BTU
Cost per 5 gallon batch = 21455.63BTUx$.00002/BTU = $.429/batch
I am sure you guys will rip my work apart, which is what I need - also, how do I calculate the amount of energy used during the boil?
i tried to do it by finding out how much heat was lost over an hour from a kettle heated to boiling then left for an hour - which I found was 55 degrees so I calculated the additional energy needed to heat the kettle that much more during the boil.
i am a terrible procrastinator -this project is due tomorrow so any corrections would be greatly appreciated.
I have the 5 page project proposal done and typed up - if I need to change a few values I can easily do so.