Thanks AJ - head hurts a bit since the mantra here has always been alkalinity and not pH in water, and here we are using an arbitrary pH of a very complex chemical and biochemical series of reactions. I do want to make sure I understand.
The basic concept that when we look at water its pH isn't nearly as important as its alkalinity still holds for approximate work. The alkalinity number in a water report is the amount of acid it takes to get to end point pH (4.3, 4.4 or 4.5 for most labs). That is completely independent of pH. I can tell you the alkalinity without saying anything about the original sample pH. It is when you ask about how much acid it takes to get to a different pH, i.e. a mash pH, that I have to know the sample pH because I need to know how much total carbo is in the sample to do that.
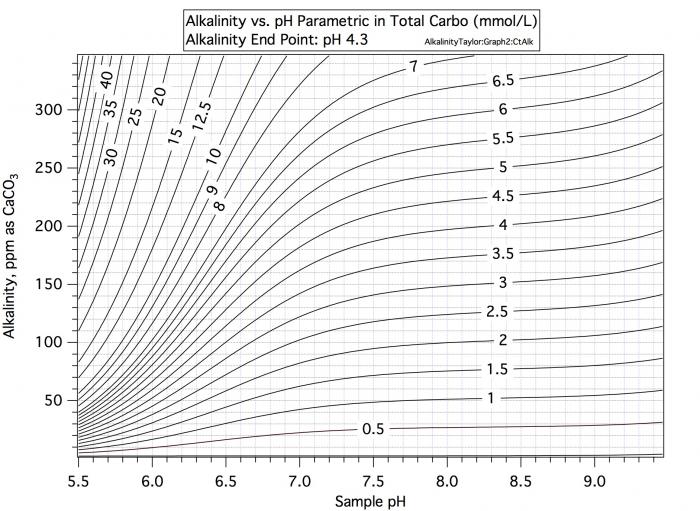
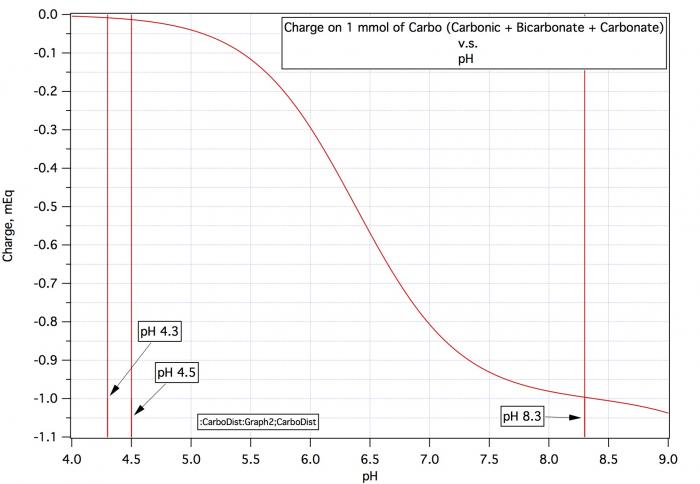
Consider a water sample with alkalinity 100 and pH 9. Consulatation of the first curve in #7 shows that this water's carbo content is 1.8, Consultation of the second curve says that if we want to take pH 9 water with its charge of about -1.04 to pH 5.4 with its charge of -0.1 /mmol we need 0.94 mEq/mmol and with 1.8 mmol/L that would be 1.69 mEq/L.
Now consider water with alkalinity 100 and pH 6.5. The first chart shows carbo content of 3.5. But the second chart shows that the charge at pH 6.5 is -0.55 so that to get to pH 5.4 with charge -0.1 you only need an acid increment of 0.45/mmol which at 3.5 mmol/L amoints to 1.58 mEq/L. Thus for a given alkalinity there must be more carbo present at lower pH to support that alkalinity but at lower pH less charge difference must be made up for by acid. These two effects cancel by and large but not completely.
Just to summarize: the suggestion is to neutralize the alkalinity...
Remember that we cannot speak of alkalinity without specifying a reference pH. We wish to neutralize (bring to 0) the alkalinity of the source water to 0
with respect to mash pH. The alkalinity,
with respect to the lab's titration end point will not have been zeroed. If we 0 WRT mash pH and send a sample to the lab they will send back a report showing the original report's alkalinity minus the alkalinity of the water
with respect to mash pH.
...of the source water through acid treatment, then add additional acid to reduce that pH (which should be low at that point) to the target mash pH (5.4 in this instance),
If you have zeroed out alkalinity
WRT mash pH then the water will be at mash pH. No further acid addition for the water will be necessary.
We wish to neutralize (bring to 0) the alkalinity of the source water to 0
with respect to mash pH. The alkalinity,
with respect to the lab's titration end point will not have been zeroed. If we 0 WRT mash pH and send a sample to the lab they will send back a report showing the original report's alkalinity minus the alkalinity of the water
with respect to mash pH.
..as well as an amount equal to bring a DI test mash to the same pH
Additional acid will be needed in the mash in order to 'neutralize' (bring to 0) the alkalinity of the base malt
with respect to mash pH. Some of this acid may come from the acidity,
with respect to mash pH, of high-kilned malts.
- so that all things are equal (pH and proton deficit). At this point, the proton deficit of the water is equal to the proton deficit of the mash
The sum of the proton deficits of each of the malts and the water is 0. The protons absorbed by the water and base malts just equal the protons given off by malt acids (and any we added).
- and consequently, the same is true for the sparge water.
As it takes 0 acid to change the pH of water which has been adjusted to mash pH to mash pH its proton deficit is 0. Adding as much of it as desired to mash at that same pH will not change the pH of the mash.
Are we ignoring hardness in this process? or is that negligible? Meaning calcium driving phosphate reactions... where calcium is likely a significant ion in the water.
In this thread we haven't talked about it but it is definitely involved. The definition of residual alkalinity makes it clear that 3.5 mEq of calcium or 7 mEq of magnesium release 1 mEq of acid. How true that is in the universe of brewing situations I don't know but until I do some experiments to check that out that's what I am using for calculations.