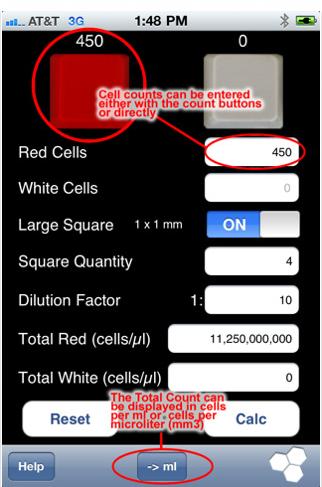
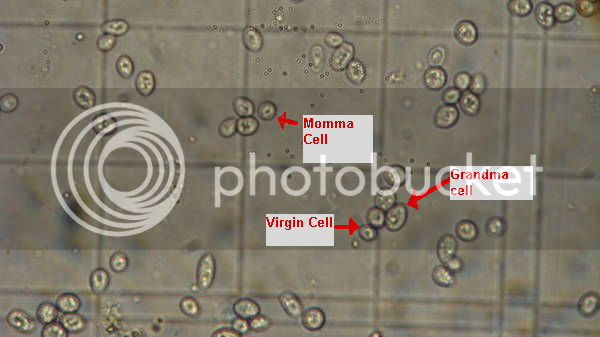
Just bought a used scope and a hemocytometer for doing yeast counts and viability measurements. Man is this cool. (eek, I think I see a bacterium... yet another thing to worry about!)
I still have to get some stains to spot the dead ones. I can see the cells easily under 400x. I took this pic with a camera pressed up to the eyepiece. Looks much better with the eyes. I don't know why the lines of the hemocytometer don't show up in the pic... they are plain as day when viewed directly.
These cells are from some very dirty BoPils yeast cake, diluted 10:1. Wyeast 2001.
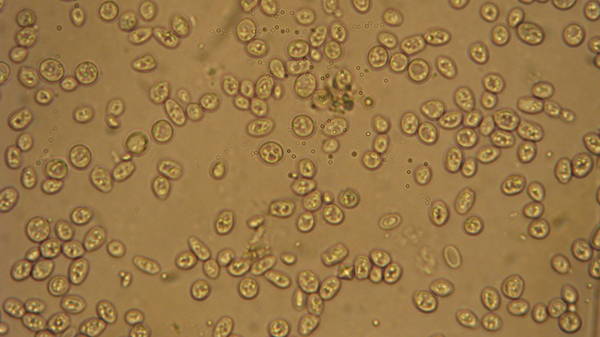
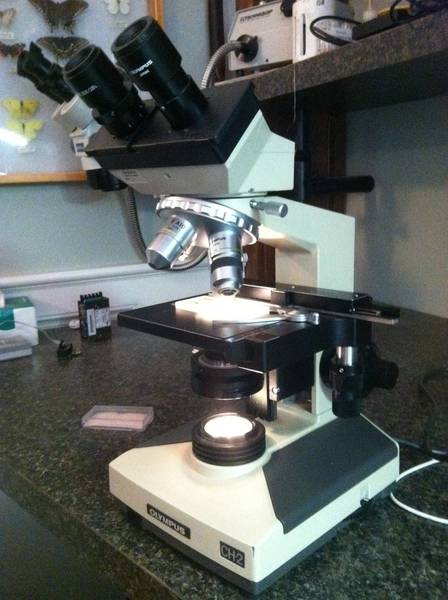
I still have to get some stains to spot the dead ones. I can see the cells easily under 400x. I took this pic with a camera pressed up to the eyepiece. Looks much better with the eyes. I don't know why the lines of the hemocytometer don't show up in the pic... they are plain as day when viewed directly.
These cells are from some very dirty BoPils yeast cake, diluted 10:1. Wyeast 2001.