Maybe some folks have this all figured out and perhaps many others don't even know it's an issue. When you ferment at one temp and then reduce the temp later for any reason, you reduce the pressure in the fermenter pretty significantly. This can be trouble when cold crashing an ale or when ramping a lager down to 35 down from a diacytel rest.
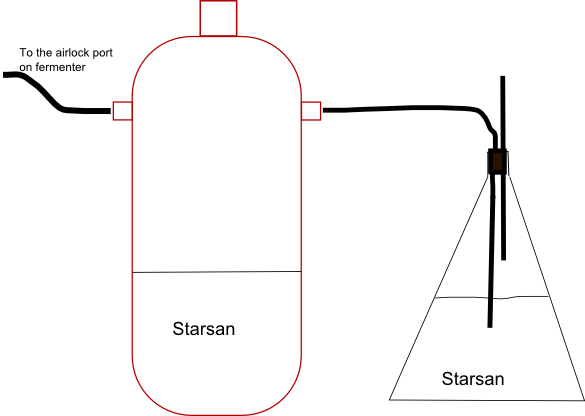
If you use a bubbler airlock, you are certainly sucking the liquid in. If you use a medium sized blowoff into some liquid, you can definitely suck that back in too. My fermenting fridge isn't exactly a spotless sanctuary and I don't like the idea of that air nor oxygen getting into the fermenter.
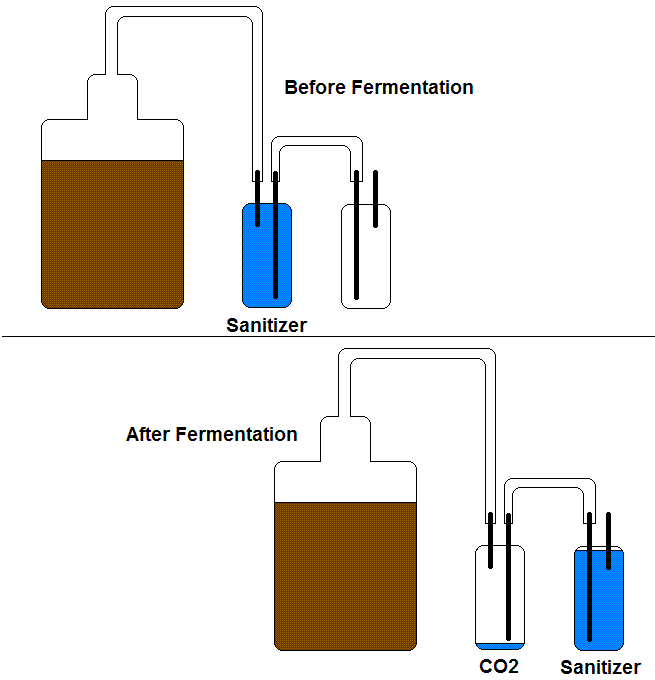
My initial idea is to fill a plastic bag with CO2 and rubberband it over the blowoff tube (the end that is normally sitting in the starsan bucket). I'd do this right when I start dropping the temp. If I anticipate it properly, an alternative is to attach a deflated bag over the carboy neck as fermentation is still happening but winding down so that it fills with "free" co2 to be sucked back in during crashing.
Discuss.
If you use a bubbler airlock, you are certainly sucking the liquid in. If you use a medium sized blowoff into some liquid, you can definitely suck that back in too. My fermenting fridge isn't exactly a spotless sanctuary and I don't like the idea of that air nor oxygen getting into the fermenter.
My initial idea is to fill a plastic bag with CO2 and rubberband it over the blowoff tube (the end that is normally sitting in the starsan bucket). I'd do this right when I start dropping the temp. If I anticipate it properly, an alternative is to attach a deflated bag over the carboy neck as fermentation is still happening but winding down so that it fills with "free" co2 to be sucked back in during crashing.
Discuss.



 Charles' Law would not make a lick of sense with a negative temperature. Should've stuck to SI units. Thanks for the info on Rankine, never looked at the absolute scale for degree F. Silly standard units
Charles' Law would not make a lick of sense with a negative temperature. Should've stuck to SI units. Thanks for the info on Rankine, never looked at the absolute scale for degree F. Silly standard units 