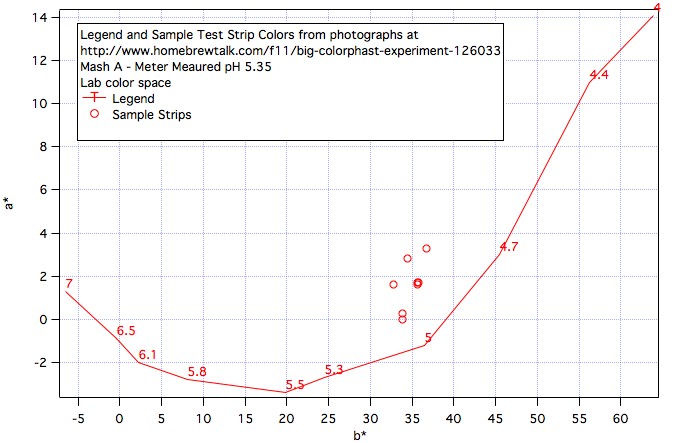
I received this interesting information from a friend of mine who spoke with a technical services representative from EMD, the producers of the ColorpHast plastic pH strips.
The Rep said that to use ColorpHast strips correctly, the strip immersion time must be from 1-10 minutes until no further change is noticed. He said that this is because of the very low ionic concentrations of what brewers measure, (ie: water/mash/wort/beer). He went on to say that the quick "Dip and Read" will NOT render a correct pH measurement. He also confirmed that the strips do read about 0.2 - 0.3 units low at mash temp. So the findings of Kai Troester and AJ Delange about the readings of these strips are confirmed.
Another thing the EMD Rep mentioned, was that ColorpHast strips have an expiration date of 3-5 years IF they are stored in a closed container with a desiccant (moisture-removing agent). Apparently the strips are adversely affected by air moisture. If the strips are not carefully stored and protected, they have a typical shelf life of 1-2 years.
Enjoy!
The Rep said that to use ColorpHast strips correctly, the strip immersion time must be from 1-10 minutes until no further change is noticed. He said that this is because of the very low ionic concentrations of what brewers measure, (ie: water/mash/wort/beer). He went on to say that the quick "Dip and Read" will NOT render a correct pH measurement. He also confirmed that the strips do read about 0.2 - 0.3 units low at mash temp. So the findings of Kai Troester and AJ Delange about the readings of these strips are confirmed.
Another thing the EMD Rep mentioned, was that ColorpHast strips have an expiration date of 3-5 years IF they are stored in a closed container with a desiccant (moisture-removing agent). Apparently the strips are adversely affected by air moisture. If the strips are not carefully stored and protected, they have a typical shelf life of 1-2 years.
Enjoy!