You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
splenda instead of priming sugar
- Thread starter megaman
- Start date

Help Support Homebrew Talk - Beer, Wine, Mead, & Cider Brewing Discussion Forum:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
Nope. but it'll be nice and sweet. Splenda (at least the regular variety) isn't fermentable.
Tankard
Well-Known Member
+ 1
Non sugar, non fermentable.
Non sugar, non fermentable.
Buy some carbonation drops. Uncap and drop the recommended dose into each. Condition all over again.
bluefoxicy
Well-Known Member
So, the batch I have in now, I used Splenda in place of the priming sugar.
...............why?
...............why?
I'm with you. Why would you ruin good beer with artifical sweetners?
Jilaman
Well-Known Member
Splenda has an interesting chemical makeup, it is indentical in chemical makeup of glucose, but it is not able to be metabolized in a biological system. It a chiral molecule, identical in makeup but not the same physically.
Anyways, no fizzy beer, only really sweet flat stuff.
There's an easy way to understand chirality. Hold out your hands, palms facing each other. Imagine that each hand is the chemical structure of a molecule. Most complex molecules are chiral. Like your hands, the two structures of chiral molecules - in sugars, they're referred to as D and L, from the Latin dexter and laevus - differ only in the arrangement of their elements. Put your hands together and they seem to match exactly. In the same way, the common sugar D-glucose is the mirror image of L-glucose, its rare counterpart. But put your hands down one on top of the other, both facing down, and you'll see that they're not identical at all; they're what chemists call non-superimposable.
Two enantiomers of a molecule will respond identically in a chemical reaction, but not so in biological systems. Proteins and cell receptors are designed to react only with particular enantiomers. For example, the enzymes in your stomach can digest only right-handed sugars. Just as a glove fits only on the proper hand, our bodies distinguish between the enantiomers of any given molecule.
Splenda usually contains 95% dextrose (the "right-handed" isomer of glucose - see dextrorotation and chirality), which the body readily metabolizes.
Anyways, no fizzy beer, only really sweet flat stuff.
Special Hops
Well-Known Member
Splenda tastes like ass. Good luck with that.
My mother-in-law used to make the best sweet tea around. turns out she is diabetic so she is now making it with Splenda and I can barely drink the stuff.
My mother-in-law used to make the best sweet tea around. turns out she is diabetic so she is now making it with Splenda and I can barely drink the stuff.
Boston Brew Guy
Well-Known Member
If he put in the equivalent amount of splenda by volume as 4.5 oz of sugar it might not be too bad. If he put in 4.5 oz of splenda, toss it because that will be overpowering.
talkingmonkey
Well-Known Member
Splenda has an interesting chemical makeup, it is indentical in chemical makeup of glucose, but it is not able to be metabolized in a biological system. It a chiral molecule, identical in makeup but not the same physically.
Anyways, no fizzy beer, only really sweet flat stuff.
Man, I just had a major flashback to organic chemistry.
I think this is a joke. Nobody would really use Splenda. Good one.
ChshreCat
Well-Known Member
Nah, they just laminate it so it doesn't get digested. 
Moonpile
Well-Known Member
I primed my last beer with Olestra™. Is that going to work?
(Note to OP: I'm really sorry. I just couldnt' resist! )
)
(Note to OP: I'm really sorry. I just couldnt' resist!
bearkluttz
Well-Known Member
megaman... you plan on replying any time soon? why did you use the splenda?
bluefoxicy
Well-Known Member
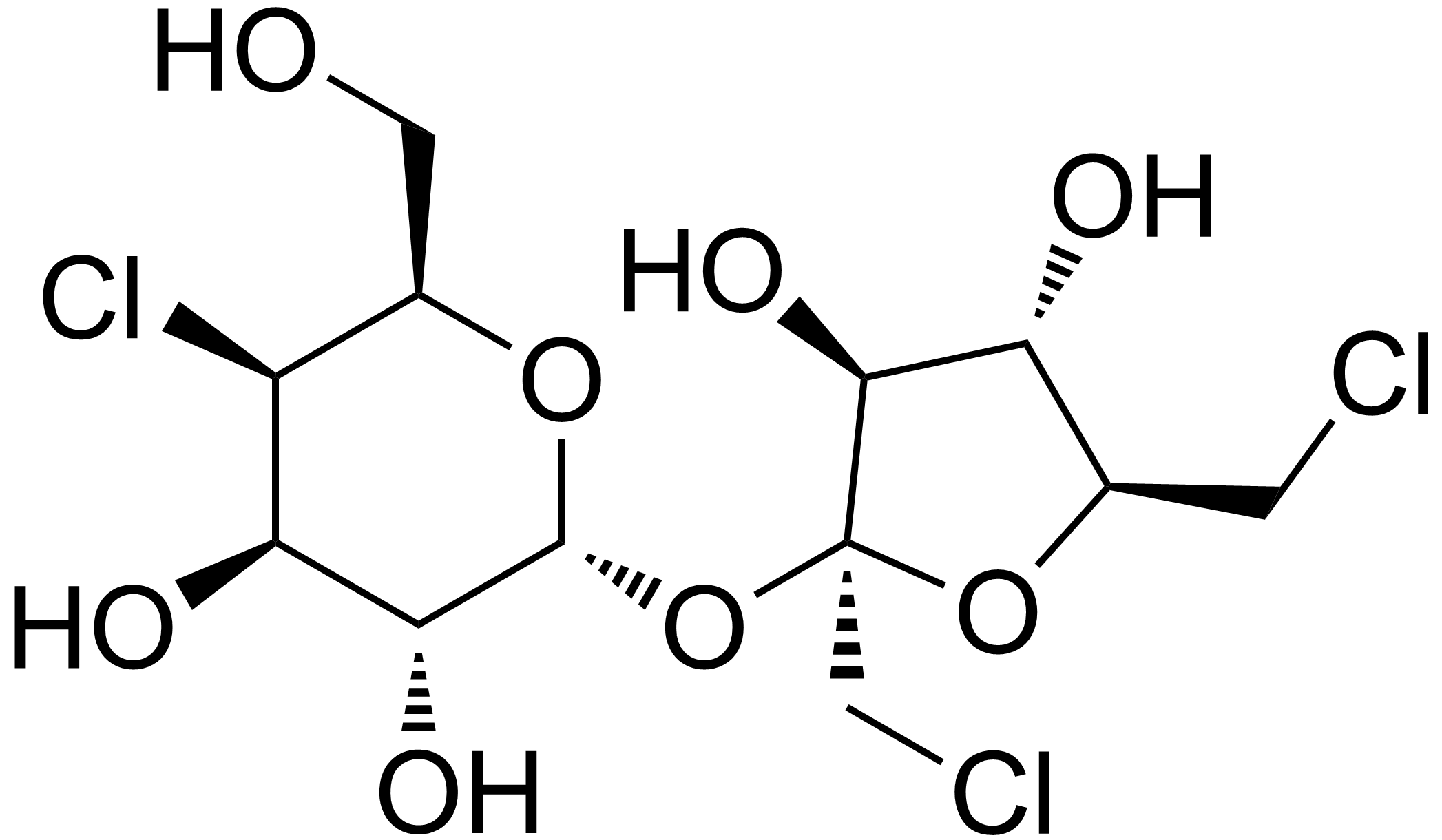
I thought sucralose was sugar bound with a couple chlorine atoms.
I thought that too. Someone told me Sucralose was sucrose bonded to a Chlorine atom "in the same way sodium is bonded to chloride in salt" and thus won't break down, to which I took up two issues:
- Sucrose has a net neutral charge; you cannot form a strong ionic bond with something having a net neutral charge, you can only form a covalent or (gasp) hydrogen bond!
- Even if it was, ionic bonds dissociate when dissolved in a polar solvent like water; sucrose is stable, such a dissociation would result in sucrose in the digestive tract!
In both cases, because of the stability of sucrose, tacking a negative ion to it would result in a good way to give yourself slow chlorine poisoning, and you better damn well drink it with green tea so the antioxidants can bond to the free chlorine atom!
Me? I won't touch any sugar substitute. It's a retarded fad, people drink tons of diet whatever "because I'm fat but I'm doing something about it" while nomming down 14 inch subs with double meat and extra cheese and mayo.
Energy drinks advertise that they're sugar free. Think about this for a minute, okay? We are going to dump tons of mild toxins and herbal extracts into your body to crank up nerve, digestive, and circulatory activity, and not supply a ready source of energy to go with it. This is like sucking half the oil out of your engine, and then blasting nitrous oxide into your air intake while racking your car up to 2000RPM past redline (it'd be like taking the fuel away, but your body won't just conk out; it'll damage itself instead).
Anyway lessons learned: don't use Splenda in beer.
Yep, I'm with ya on the sugar substitutes. I don't trust any of them. (This makes me want to rant a little that Wrigley has put sugar substitutes in every one of its chewing gum products, and now I don't use them. I miss Juicy Fruit. <sigh>)
Gave up margarine in favor of butter too. Woot!
</off topic>
Gave up margarine in favor of butter too. Woot!
</off topic>
knownikko
Well-Known Member
I thought sucralose was sugar bound with a couple chlorine atoms.
Not quite. It's actually a sucrose molecule that has three of its hydroxyl groups replaced with chlorine atoms, which keeps the molecule stable.
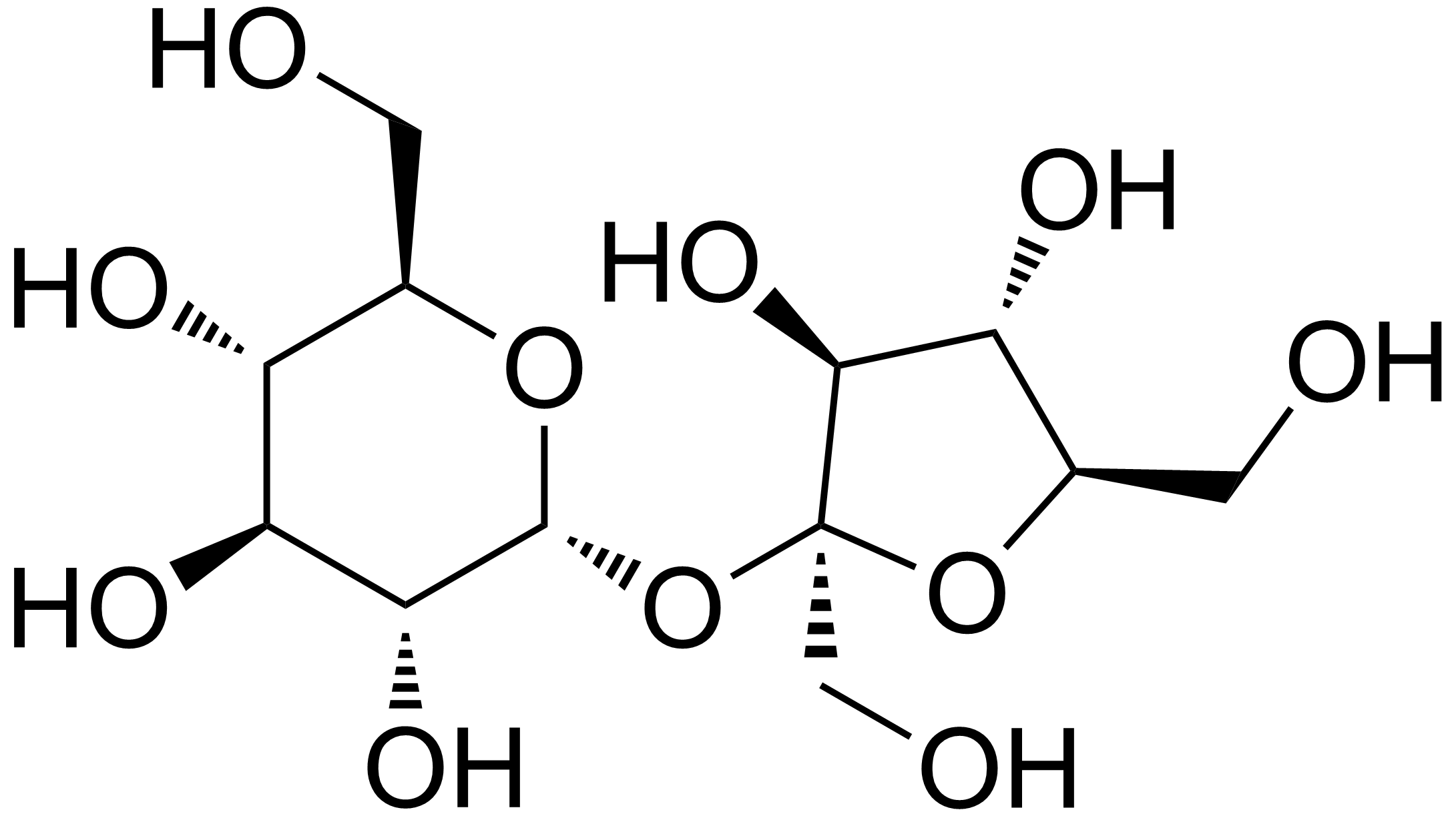
From Wikipedia:
Sucralose (Splenda):
Sucrose (table sugar):
Sucralose - Wikipedia, the free encyclopedia
Similar threads
- Replies
- 4
- Views
- 711
- Replies
- 4
- Views
- 491

